Abstract
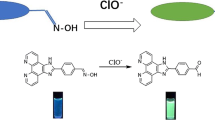
In this study, the synthesis of 7-((Hydroxyimino)methyl)-1,10-phenanthroline-4-carbaldehyde oxime (1) in two steps starting from 4,7-dimethyl-1,10-phenanthroline (2) is reported. It is found that compound 1 can be used as a fluorogenic probe for the detection of hypochlorite ion in aqueous solution. NMR and mass spectral analysis indicate that probe 1 undergoes a chemical transformation through its oxime units upon treatment with hypochlorite, which results in a remarkable enhancement of the emission intensity. Also, metal ion recognition properties of probe 1 is investigated. It is noted that compound 1 is responsive to Zn2+, Cd2+, Ni2+ and Cu2+ metal ions, which reduced the emission intensity under identical conditions.

The design, synthesis and properties of a new fluorescent hypochlorite probe is described. It is found that probe 1 immediately undergoes an oxidation reaction with NaClO through its oxime units in 0.1 M Na2CO3-NaHCO3 buffer containing DMF (pH = 9.0, 30:1 v/v) at room temperature, which resulted in a remarkable enhancement of the emission intensity. It is noteworthy that this novel probe 1 is highly selective to hypochlorite ion when compared to some other ROS and anions. On the other hand, probe 1 also induces turn-off fluorogenic responses to metal ions such as Zn2+, Cd2+, Ni2+ and Cu2+ ions under identical conditions.
















Similar content being viewed by others
References
Doura T, Nonakaa H, Sando S (2012) Atom arrangement strategy for designing a turn-on 1 H magnetic resonance probe: a dual activatable probe for multimodal detection of hypochlorite. Chem Commun 48:1565–1567
Liu F, Wu T, Cao J, Zhang H, Hu M, Sun S, Song F, Fan J, Wang J, Peng X (2013) A novel fluorescent sensor for detection of highly reactive oxygen species, and for imaging such endogenous hROS in the mitochondria of living cells. Analyst 138:775–778
Pattison DI, Davies MJ (2006) Evidence for rapid inter- and intramolecular chlorine transfer reactions of histamine and carnosine chloramines: implications for the prevention of hypochlorous-acid-mediated damage. Biochemistry 45:8152–8162
Lin W, Long L, Chen B, Tan W (2009) A ratiometric fluorescent probe for hypochlorite based on a deoximation reaction. Chem Eur J 15:2305–2309
Aokl T, Munemorl M (1983) Continucus flow determination of free chlorine in water. Anal Chem 55:209–212
Long L, Zhang D, Li X, Zhang J, Zhang C, Zhou L (2013) A fluorescence ratiometric sensor for hypochlorite based on a novel dual-fluorophore response approach. Anal Chim Acta 775:100–105
Wang Q, Liu C, Chang J, Lu Y, He S, Zhao L, Zeng X (2013) Novel water soluble styrylquinolinium boronic acid as a ratiometric reagent for the rapid detection of hypochlorite ion. Dyes Pigments 99:733–739
Shi J, Li Q, Zhang X, Peng M, Qin J, Li Z (2010) Simple triphenylamine-based luminophore as a hypochlorite chemosensor. Sensors Actuators B 145:583–587
Huoa FJ, Zhang JJ, Yang YT, Chao JB, Yin CX, Zhang YB, Chen TG (2012) A fluorescein-based highly specific colorimetric and fluorescent probe for hypochlorites in aqueous solution and its application in tap water. Sensors Actuators B 166–167:44–49
Goswami S, Das AK, Manna A, Maity AK, Saha P, Quah CK, Fun HK, Abdel-Aziz HA (2014) Nanomolar detection of hypochlorite by a rhodamine-based chiral hydrazide in absolute aqueous media: application in tap water analysis with live-cell imaging. Anal Chem 86:6315–6322
Lou X, Zhang Y, Li Q, Qin J, Li Z (2011) A highly specific rhodamine-based colorimetric probe for hypochlorites: a new sensing strategy and real application in tap water. Chem Commun 47:3189–3191
Cheng X, Jia H, Long T, Feng J, Qin, J, Li Z (2011) A “turn-on” fluorescent probe for hypochlorous acid: convenient synthesis, good sensing performance, and a new design strategy by the removal of C=N isomerization. Chem Commun 47:11,978–11,980.
Goswami S, Manna A, Paul S, Quahb CK, Fun HK (2013) Rapid and ratiometric detection of hypochlorite with real application in tap water: molecules to low cost devices (TLC sticks). Chem Commun 49:11,656–11,658
Goswami S, Aich K, Das S, Pakhira B, Ghoshal K, Quah CK, Bhattacharyya M, Fun H. K, Sarkar S (2015) A Triphenyl Amine-Based Solvatofluorochromic Dye for the Selective and Ratiometric Sensing of OCl− in Human Blood Cells. Chem Asian J 10:694–700.
Koide Y, Urano Y, Hanaoka K, Terai T, Nagano T (2011) Development of an Si-Rhodamine-Based Far-Red to Near-Infrared Fluorescence Probe Selective for Hypochlorous Acid and Its Applications for Biological Imaging. J Am Chem Soc 133:5680–5682
Xu Q, Lee KA, Lee S, Lee KM, Lee WJ, Yoon J (2013) A Highly Specific Fluorescent Probe for Hypochlorous Acid and Its Application in Imaging Microbe-Induced HOCl Production. J Am Chem Soc 135:9944–9949
Manjare ST, Lee JKY, Churchill DG (2014) Facile meso-BODIPY Annulation and Selective Sensing of Hypochlorite in Water. Org Lett 16:520–523
Yang YK, Cho HJ, Shin JLI, Tae J (2009) A Rhodamine − Hydroxamic Acid-Based Fluorescent Probe for Hypochlorous Acid and Its Applications to Biological Imagings. Org Lett 11:859–861
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Signaling Recognition Events with Fluorescent Sensors and Switches. Chem Rev 97:1515–1566
de Silva AP, Fox DB, Huxley AJM (2000) Combining Fluorescence, Coordination Chemistry and Electron Transfer for Signalling Purposes. Coord Chem Rev 205:41–57
Callan JF, de Silva AP, Magri DC (2005) Luminescent sensors and switches in the early 21st century. Tetrahedron 61:8551–8588
Kim JS, Quang DT (2007) Calixarene-Derived Fluorescent Probes. Chem Rev 107:3780–3799
Coskun A, Akkaya EU (2005) Ion Sensing Coupled to Resonance Energy Transfer: A Highly Selective and Sensitive Ratiometric Fluorescent Chemosensor for Ag(I) by a Modular Approach. J Am Chem Soc 127:10,464–10,465
Ozlem S, Akkaya EU (2009) Thinking Outside the Silicon Box: Molecular AND Logic As an Additional Layer of Selectivity in Singlet Oxygen Generation for Photodynamic Therapy. J Am Chem Soc 13:48–49
Guliyev R, Coskun A, Akkaya EU (2009) Design Strategies for Ratiometric Chemosensors: Modulation of Excitation Energy Transfer at the Energy Donor Site. J Am Chem Soc 13:9007–9013
Atilgan S, Ozdemir T, Akkaya EU (2010) Selective Hg(II) Sensing with Improved Stokes Shift by Coupling the Internal Charge Transfer Process to Excitation Energy Transfer. Org Lett 12:4792–4795
Isik M, Ozdemir T, Turan IS, Kolemen S, Akkaya EU (2013) Chromogenic and Fluorogenic Sensing of Biological Thiols in Aqueous Solutions Using BODIPY-Based Reagents. Org Lett 15:216–219
Turan IS, Akkaya EU (2014) Chemiluminescence Sensing of Fluoride Ions Using a Self-Immolative Amplifier. Org Lett 16:1680–1683
Atilgan N, Algi F, Cihaner A, Önal AM (2009) Synthesis and properties of a novel redox driven chemiluminescent material built on a terthienyl system. Tetrahedron 65:5776–5781
Asil D, Cihaner A, Algi F, Önal AM (2010) A Diverse-Stimuli Responsive Chemiluminescent Probe with Luminol Scaffold and Its Electropolymerization. Electroanalysis 22:2254–2260
Algi M. P, Öztaş Z, Algi F (2012) Triple channel responsive Cu2+ probe. Chem Commun 48:10,219–10,221.
Algi F, Cihaner A (2008) An electroactive polymeric material and its voltammetric response towards alkali metal cations in neat water. Tetrahedron Lett 49:3530–3533
Pamuk M, Algi F (2012) Incorporation of a 2,3-dihydro-1H-pyrrolo[3,4-d]pyridazine-1,4(6H)-dione unit into a donor–acceptor triad: synthesis and ion recognition features. Tetrahedron Lett 53:7117–7120
Öztaş Z, Pamuk M, Algi F (2013) Nonreaction-based fluorescent Au3+ probe that gives fast response in aqueous solution. Tetrahedron 69:2048–2051
Degirmenci A, Iskenderkaptanoglu D, Algi F (2015) A novel turn-off fluorescent Pb(II) probe based on 2,5-di(thien-2-yl)pyrrole with a pendant crown ether. Tetrahedron Lett 56:602–607
Accorsi G, Listorti A, Yoosaf K, Armaroli N (2009) 1,10-Phenanthrolines: versatile building blocks for luminescent molecules, materials and metal complexes. Chem Soc Rev 38:1690–1700
Kaur N, Alreja P (2015) A novel 1,10-phenanthroline based chemosensor for differential metal ion sensing and constructing molecular logic gates. Tetrahedron Lett 56:182–186
Yang C, Xu J, Chen W, Lu M, Li Y, Wang XJ (2014) A novel colorimetric and fluorescent sensor for fluoride detection based on a three-arm phenanthroline derivative. J Mater Sci 49:7040–7048
Pamuk M, Algi F (2012) Synthesis of a novel on/off fluorescent cadmium(II) probe. Tetrahedron Lett 53:7010–7012
Karakaya S, Algi F (2014) A novel dual channel responsive zinc(II) probe. Tetrahedron Lett 55:5555–5559
Yoldas A, Algi F (2015) An imidazo-phenanthroline scaffold enables both chromogenic Fe(II) and fluorogenic Zn(II) detection. RSC Advances 5:7868–7873
Goswamia S, Maitya S, Maitya AC, Das AK (2014) Fluorometric and naked-eye detectable dual signaling chemodosimeter for hypochlorite. Sensors Actuators B 204:741–745
Wu X, Li Z, Yang L, Hanb J, Han S (2013) A self-referenced nanodosimeter for reaction based ratiometric imaging of hypochlorous acid in living cells. Chem Sci 4:460–467
Emrullahoğlu M, Üçüncü M, Karakuş E (2013) A BODIPY aldoxime-based chemodosimeter for highly selective and rapid detection of hypochlorous acid. Chem Commun 49:7836–7838
Boone LL (2006) The highly preorganized ligands 1,10-Phenanthroline-2,9-dialdoxime and bis-1,10-Phenanthroline, and their complexing properties with metal ions. University of North Carolina Wilmington, Dissertation Thesis
Williams NJ, Dean NE, VanDerveer DG, Luckay RC, Hancock RD (2009) Strong Metal Ion Size Based Selectivity of the Highly Preorganized Ligand PDA (1,10 Phenanthroline-2,9-dicarboxylic Acid) with Trivalent Metal Ions. A Crystallographic, Fluorometric, and Thermodynamic Study. Inorg Chem 48:7853–7863
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic ditances in halides and chalcogenides. Acta Crystallogr Sect A A32:751–767
Acknowledgments
The author is indebted to Aksaray University for partial financial support of this work (ASU BAP 2015-092).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 1.62 mb)
Rights and permissions
About this article
Cite this article
Algi, M.P. A Fluorescent Hypochlorite Probe Built on 1,10-Phenanthroline Scaffold and its Ion Recognition Features. J Fluoresc 26, 487–496 (2016). https://doi.org/10.1007/s10895-015-1734-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1734-7