Abstract
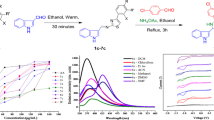
The inclusion complexes of a series of 4-arylidenamino-5-phenyl-4H-1, 2, 4-triazole-3-thiols have been prepared with β-cyclodextrin. The compounds and their inclusion complexes have been characterized by studying their physical and spectral properties. The thermodynamic stability constant and free energy of activation have been determined to know the stability of inclusion complexes and type of host-guest relation. Finally, absorption, excitation and emission spectra of the compounds (4-arylidenamino-5-phenyl-4H-1, 2, 4-triazole-3-thiols) and their inclusion complexes have been taken. It is found that inclusion complex formation brings about a drastic change in absorption and fluorescence characteristic (both excitation and emission spectra) of newly synthesized compounds.















Similar content being viewed by others
References
Ayman A, Shafi A, Shar S, Shihry AL (2009) Fluorescence enhancement of 1-napthol-5-sulfonate by forming inclusion complex with β- cyclodextrin in aqueous solution. Spectrochim Acta A Mol Biomol Spectrosc 72(3):533–537
Muthu Vijayan Enoch IV, Swaminathan M (2004) Inclusion complexation of 2-amino-7-bromofluorene by β-cyclodextrin: spectral characteristics and the effect of PH. J Fluoresc 14:751–756
Wang R, Yuan L, Donal HM (2005) A green to blue fluorescence switch of protonated 2-aminoanthracene upon inclusion in cucurbit[7]uril. Chem Commun 47:5867–5869
Maafi M, Laassis B, Aaron JJ (1995) Photochemically induced fluoroscence investigation of a β-cyclodextrin: azure a inclusion complex and determination of analytical parameters. J Inclusion Phenom Mol Recognit Chem 22:235–247
Misiuk W (2012) Spectrofluorimetric study on inclusion interaction of β- cyclodextrin with duloxetine and its analytical application. Ind J Chem 51A:1706–1710
Ahmed RM, Dani M, Elbashir AA (2013) Host–guest inclusion complex of β-cyclodextrin and cephalexin and its analytical application. Int J Pharm Chem Res 2:2278–2290
Nelson G, Patonay G, Warner IM (1988) Effects of selected alcohols on cyclodextrin inclusion complexes of pyrene using fluorescence lifetime measurements. Anal Chem 60:274–279
Shaker RM (2006) The chemistry of mercapto- and thione- substituted 1,2,4-triazole and their utility in heterocyclic synthesis. ARKIVOC 2006(ix):59–112
Bayrak H, Demirbas A, Demirbas N, Karaoglu SA (2009) Synthesis of some new 1,2,4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities. Eur J Med Chem 44(11):4362–4366
Xu J, Cao Y, Zhang J (2011) Design, synthesis and antifungal activities of novel 1,2,4-triazole derivatives. Eur J Med Chem 46(7):3142–3148
Pintilie O, Profire L, Sunel V, Popa M, Pui A (2007) Synthesis and antimicrobial activity of some new 1,3,4-thiadiazole and 1,2,4-triazole compounds having a D, L-methionine moiety. Molecules 12:103–113
Bayrak H, Demirbas A, Karaoglu SA, Demirbas N (2009) Synthesis of some new 1,2,4-triazoles, their mannich and Schiff bases and evaluation of their antimicrobial activities. Eur J Med Chem 44:1057–1066
Holla BS, Veerandra B, Shivananda MK, Poojary B (2003) Synthesis characterization and anticancer activity studies on some mannich bases derived from 1,2,4-triazoles. Eur J Med Chem 38(7–8):759–767
Al-Soud YA, Al-Masoudi NA, AE-RS (2006) Ferwanah synthesis and properties of new substituted 1,2,4-triazoles: potential antitumor agents. Bioorg Med Chem 11: 1701–1708
Abdel-Aal MT, El-Sayed WA, El-Kosy SM, El-Ashry ESH (2008) Synthesis and antiviral evaluation of novel 5-(N-arylaminomethyl-1,3,4-oxadiazol-2-yl)hydrazines and their sugars,1,2,4-triazoles, tetrazoles and pyrazolyl derivatives. Arch Pharm 341(5):307–313
Labanauskas L, Udrenaite E, Gaidelis P, Brukstus A (2004) Synthesis of 5-(2-,3- and 4-methoxyphenyl)-4 H-1,2,4-triazole-3-thiol derivatives exhibiting anti-inflammatory activity. IlFarmaco 59(4):255–259
Labanauskas L, Kalcas V, Uderenaite E, Gaidelis P, Brukstus A, Dauksas V (2001) Synthesis of 3-(3,4-dimethoxyphenyl)-1 H-1,2,4-triazole-5-thiol and 2-amino-5-(3,4-dimethoxyphenyl)-1,3,4-thiadiazole derivatives exhibiting anti-inflammatory activity. Pharmazie 56:617–619
Almasirad SA, Tabatabai M (2004) Synthesis and anticonvulsant activity of new 2-substituted-5-[2-(2-fluorophenoxy) phenyl]-1,3,4-oxadiazoles and 1,2,4-triazoles. Bioorg Med Chem Lett 14(24):6057–6059
Yang B, Lin J, Chen Y, Liu Y (2009) Artemether/hydroxypropyl-b-cyclodextrin host-guest system: characterization, phase-solubility and inclusion mode. Bioorg Med Chem 17:6311–6317
Panda S, Sahu R (2013) Studies on inclusion complex of 3-(2-chloro phenyl) 4-methyl 2- thiocarbamoyl-3,3a-dihydro pyrazolo[3,4c] pyrazole. Am J Adv Drug Deliv 1(5):1–9
Panda S, Sahu R (2014) Studies on inclusion complex of 4-methyl-3-phenyl-2- thiocarbamoyl-3,3a-dihydro pyrazolo[3,4c] pyrazole. J Pharm Bio Sci 9(2):30–36
Panda S, Singh DL (2014) Study of antioxidant, antimicrobial and anthelmintic properties of 1-nicotinoyl-4-aryl-3-methyl 3a,4-dihydropyrazolo [3,4c] pyrazoles and their inclusion complexes with β-cyclodextrin. World J Pharm Pharma Sci 3(4):1639–1654
Panda S, Singh DL, Sahu R (2013) Study of antioxidant properties of 1-nicotinoyl-4-aryl-3-methyl 3a,4-dihydropyrazole [3,4c] pyrazoles and their inclusion complexes with β-cyclodextrin. Int J Chem Pharm Sci 4(2):1–6
Panda S, Nayak S, Das PK (2015) Impact of inclusion complex formation on antioxidant activity of some 4-arylidenamino-5-phenyl-4h-1, 2, 4-triazole-3-thiols. World J Pharm Pharm Sci 4:939–951
Higuchi T, Connors K (1965) Phase -solubility technique. Adv Anal Chem Instrum 4:117–212
Ueda H, Endo T (2006) Large-ring cyclodextrins. In: Dodziuk H (ed) cyclodextrins and their complexes. Chemistry, analytical methods, applications. Wiley-VCH Verlag, Weinheim, pp. 370–380
Bensi HA, Hilderband JH (1999) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707
Szetli J (1985) Molecular entrapment and release properties of drugs by cyclodextrins. Controlled drug Bio-availability. Vol. 3, Willey interscience publications, New York, pp 365–420
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panda, S., Nayak, S. Studies on Absorption and Emission Characteristics of Inclusion Complexes of Some 4-Arylidenamino-5-phenyl-4H-1, 2, 4-triazole-3-thiols. J Fluoresc 26, 413–425 (2016). https://doi.org/10.1007/s10895-015-1728-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1728-5