Abstract
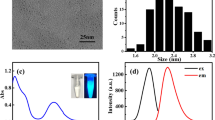
A new Cd-Cysteine complex nanorods (Cd-Cys NRs) were synthesized in one step at room temperature, and its morphology, structure and spectral properties were characterized by transmission electron microscopy (TEM), elemental analysis (EA), X-Ray diffraction (XRD), solid state and normal UV-Vis, Fourier transform infrared (FTIR) and spectrofluorometry. The developed Cd-Cys NRs were used as a fluorescence sensor for detection of Fe (III) in different aqueous matrices. The selectivity and sensitivity of the fabricated nano-sensor based on its fluorescence quenching in the presence of Fe (III) were probed according to the Stern-Volmer equation. The detection limit of the method was in micro-molar per liter range. Cd-Cys NRs response tested in different complex samples such as Rosemary plant leaves, exhibited a well-defined response. Anticoagulation measurements were performed to evaluate their blood biocompatibility.

ᅟ














Similar content being viewed by others
References
Jung HS, Han JH, Pradhan T, Kim S, Lee SW, Sessler JL, Kim TW, Kang C, Kim JS (2012) A cysteine-selective fluorescent probe for the cellular detection of cysteine. Biomaterials 33(3):945–953
Kim JS, Quang DT (2007) Calixarene-derived fluorescent probes. Chem Rev 107(9):3780–3799
De Silva AP, Gunaratne HN, Gunnlaugsson T, Huxley AJ, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97(5):1515–1566
Jung HS, Kwon PS, Lee JW, Kim JI, Hong CS, Kim JW, Yan S, Lee JY, Lee JH, Joo T (2009) Coumarin-derived Cu2+−selective fluorescence sensor: synthesis, mechanisms, and applications in living cells. J Am Chem Soc 131(5):2008–2012
Neupane LN, Park J-Y, Park JH, Lee K-H (2013) Turn-on fluorescent chemosensor based on an amino acid for Pb (II) and Hg (II) ions in aqueous solutions and role of tryptophan for sensing. Org Lett 15(2):254–257
Han A, Liu X, Prestwich GD, Zang L (2014) Fluorescent sensor for Hg2+ detection in aqueous solution. Sensors Actuators B Chem 198:274–277
Zhong K, Cai M, Hou S, Bian Y, Tang L (2014) A simple benzimidazole based fluorescent sensor for ratiometric recognition of Zn2+ in water. Bull Kor Chem Soc 35(2):489
Hu SH, Liu DM, Tung WL, Liao CF, Chen SY (2008) Surfactant-free, self-assembled PVA-iron oxide/silica core–shell nanocarriers for highly sensitive, magnetically controlled drug release and ultrahigh cancer cell uptake efficiency. Adv Funct Mater 18(19):2946–2955
Kawakubo S, Hagihara Y, Honda Y, Iwatsuki M (1999) Speciation of iron in river and tap waters by catalytic spectrophotometry using oxidation of o-phenylenediamine with hydrogen peroxide. Anal Chim Acta 388(1):35–43
Niazi A, Zolgharnein J, Davoodabadi MR (2007) Simultaneous determination of aluminium and iron with hematoxylin using spectrophotometric and orthogonal signal correction-partial least squares in plant and water. Ann Chim 97(11–12):1181–1190
Brinkmann M, Teuffel R, Laham N, Ehrlich R, Decker P, Lemonnier FA, Pascolo S (2007) Expression of iron transport proteins divalent metal transporter-1, Ferroportin-1, HFE and transferrin receptor-1 in human monocyte-derived dendritic cells. Cell Biochem Funct 25(3):287–296
Locatelli C (2004) Heavy metals in matrices of food interest: sequential voltammetric determination at trace and ultratrace level of copper, lead, cadmium, zinc, arsenic, selenium, manganese and iron in meals. Electroanalysis 16(18):1478–1486
Unnithan MR, Vinod V, Anirudhan T (2002) Ability of iron (III)-loaded carboxylated polyacrylamide-grafted sawdust to remove phosphate ions from aqueous solution and fertilizer industry wastewater: adsorption kinetics and isotherm studies. J Appl Polym Sci 84(13):2541–2553
Hunt RC, Davis AA (1992) Release of iron by human retinal pigment epithelial cells. J Cell Physiol 152(1):102–110
Safavi A, Abdollahi H (2002) Simultaneous kinetic determination of Fe (III) and Fe (II) by H-point standard addition method. Talanta 56(4):699–704
Costa RCC, Araújo AN (2001) Determination of Fe (III) and total Fe in wines by sequential injection analysis and flame atomic absorption spectrometry. Anal Chim Acta 438(1):227–233
van den Berg CM (2006) Chemical speciation of iron in seawater by cathodic stripping voltammetry with dihydroxynaphthalene. Anal Chem 78(1):156–163
Andersen JE (2005) A novel method for the filterless preconcentration of iron. Analyst 130(3):385–390
Arnold GL, Weyer S, Anbar A (2004) Fe isotope variations in natural materials measured using high mass resolution multiple collector ICPMS. Anal Chem 76(2):322–327
Huber JK (1999) Determination of Cu, Fe, Mn, and Zn in blood fractions by SEC-HPLC-ICP-AES coupling. Analyst 124(5):657–663
Singh N, Kaur N, Dunn J, MacKay M, Callan JF (2009) A new fluorescent chemosensor for iron (III) based on the β-aminobisulfonate receptor. Tetrahedron Lett 50(8):953–956
Mao J, He Q, Liu W (2010) An rhodamine-based fluorescence probe for iron (III) ion determination in aqueous solution. Talanta 80(5):2093–2098
Tripathy SK, Woo JY, Han C-S (2013) Colorimetric detection of Fe (III) ions using label-free gold nanoparticles and acidic thiourea mixture. Sensors Actuators B Chem 181:114–118
Liu X-L, Zhu Y-J (2009) A precursor nanowire templated route to CdS nanowires. Mater Lett 63(12):1085–1088
Xiong S, Xi B, Xu D, Wang C, Feng X, Zhou H, Qian Y (2007) L-cysteine-assisted tunable synthesis of PbS of various morphologies. J Phys Chem C 111(45):16761–16767
Xiang J, Cao H, Wu Q, Zhang S, Zhang X, Watt AA (2008) L-cysteine-assisted synthesis and optical properties of Ag2S nanospheres. J Phys Chem C 112(10):3580–3584
Chen X, Zhou Y, Peng X, Yoon J (2010) Fluorescent and colorimetric probes for detection of thiols. Chem Soc Rev 39(6):2120–2135
Lill R, Mühlenhoff U (2006) Iron-sulfur protein biogenesis in eukaryotes: components and mechanisms. Annu Rev Cell Dev Biol 22:457–486
Chen Y, Rosenzweig Z (2002) Luminescent CdS quantum dots as selective ion probes. Anal Chem 74(19):5132–5138
Tang Z, Kotov NA, Giersig M (2002) Spontaneous organization of single CdTe nanoparticles into luminescent nanowires. Science 297(5579):237–240
Yong S-M, Muralidharan P, Jo SH, Kim DK (2010) One-step hydrothermal synthesis of CdTe nanowires with amorphous carbon sheaths. Mater Lett 64(14):1551–1554
Chua JH, Chee R-E, Agarwal A, Wong SM, Zhang G-J (2009) Label-free electrical detection of cardiac biomarker with complementary metal-oxide semiconductor-compatible silicon nanowire sensor arrays. Anal Chem 81(15):6266–6271
You HS, Choi KS, Bae PK, Kim KN, Jang HG, Kim Y-R, Kim CH (2009) Preparation and characterization of various surface-modified semiconductor nanocrystals. Notes 30(12):3137
Huang X-J, Choi Y-K (2007) Chemical sensors based on nanostructured materials. Sensors Actuators B Chem 122(2):659–671
Yoe JH, Jones AL (1944) Colorimetric determination of iron with disodium-1, 2-dihydroxybenzene-3, 5-disulfonate. Ind Eng Chem Anal Ed 16(2):111–115
Liu X-L, Zhu Y-J, Zhang Q, Li Z-F, Yang B (2012) Cd–cysteine precursor nanowire templated microwave-assisted transformation route to CdS nanotubes. Mater Res Bull 47(12):4263–4265
Parsons JG, Dokken KM, McClure J, Gardea-Torresdey JL (2013) FTIR, XAS, and XRD study of cadmium complexes with l-cysteine. Polyhedron 56:237–242
Monshi A, Foroughi MR, Monshi MR (2012) Modified Eq. 2 to estimate more accurately Nano-crystallite size using XRD. World J Nano Sci Eng 2:154
Pretsch E, Clerc T, Seibl J, Simon W (1989) Tables of spectral data for structure determination of organic compounds. Springer, Berlin, pp 205–210
Giusti MM, Wrolstad RE (2001) Characterization and measurement of anthocyanins by UV‐visible spectroscopy. Current protocols in food analytical chemistry. Wiley, New York, pp 150–180
An X, Cao C, Yu X (2010) Fabrication of biocompatible Zn–cysteine nanowires and their application in selective fluorescence detection of Cu2+. J Nanosci Nanotechnol 10(12):8356–8361
Doak J, Gupta R, Manivannan K, Ghosh K, Kahol P (2010) Effect of particle size distributions on absorbance spectra of gold nanoparticles. Phys E Low-Dim Syst Nanostruct 42(5):1605–1609
Martinez CE, McBride MB (1998) Coprecipitates of Cd, Cu, Pb and Zn in iron oxides: solid phase transformation and metal solubility after aging and thermal treatment. Clay Clay Miner 46(5):537–545
Patoczka J, Johnson R, Scheri J (1998) Trace heavy metals removal with ferric chloride. In: Proceedings of Water Environment Federation Industrial Wastes Technical Conference, Nashville, TN. pp 1–14
Weerakkody NS, Caffin N, Lambert LK, Turner MS, Dykes GA (2011) Synergistic antimicrobial activity of galangal (Alpinia galanga), rosemary (Rosmarinus officinalis) and lemon iron bark (Eucalyptus staigerana) extracts. J Sci Food Agric 91(3):461–468
Kaloustian J, Portugal H, Pauli A, Pastor J (2002) Chemical, chromatographic, and thermal analysis of rosemary (Rosmarinus officinalis). J Appl Polym Sci 83(4):747–756
Huang N, Yang P, Leng Y, Chen J, Sun H, Wang J, Wang G, Ding P, Xi T, Leng Y (2003) Hemocompatibility of titanium oxide films. Biomaterials 24(13):2177–2187
Radojevic M, Bashkin VN (1999) Practical environmental analysis. Royal Society of Chemistry, London
Liang Z-Q, Wang C-X, Yang J-X, Gao H-W, Tian Y-P, Tao X-T, Jiang M-H (2007) A highly selective colorimetric chemosensor for detecting the respective amounts of iron (II) and iron (III) ions in water. New J Chem 31(6):906–910
Acknowledgments
We would like to thank Mr. Bahman Mansouri Motlagh for his special helps on anticoagulation measurements and blood sample analyzing, and University of Birjand Research Council for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 151 kb)
Rights and permissions
About this article
Cite this article
Ghiamati, E., Boroujerdi, R. Cd-Cysteine Nanorods as a Fluorescence Sensor for Determination of Fe (III) in Real Samples. J Fluoresc 26, 135–147 (2016). https://doi.org/10.1007/s10895-015-1693-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1693-z