Abstract
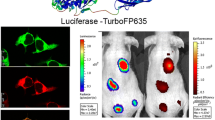
By tracking reporter molecules such as green fluorescent protein and luciferase, researchers can determine physiological status and follow processes both in vitro and in vivo. Here, we describe a dual-reporter imaging method, in which a fusion of eGFP and Luc2 is introduced into hosts using lentiviral particles based on HIV-1. The fusion molecule is both fluorescent and bioluminescent, and is therefore ideal as an optical marker in clinical and research applications. We characterized multiple technical indices of the molecule, including sensibility, biocompatibility, lifetime, and others. Lentiviral particles carrying the reporter were strongly infective in endothelial progenitor (EPC) and GL261 glioma cells, as well as in live mice. By transforming Luc2-eGFP into hosts, morphological and quantitative data can be collected not only from tissue specimens but also from live animal models.




Similar content being viewed by others
References
Kost B, Spielhofer P, Chua NH (1998) A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J: Cell Mol Biol 16(3):393–401
Dhonukshe P, Laxalt AM, Goedhart J, Gadella TW, Munnik T (2003) Phospholipase d activation correlates with microtubule reorganization in living plant cells. Plant Cell 15(11):2666–2679. doi:10.1105/tpc.014977
Mitsiades CS, Mitsiades NS, Bronson RT, Chauhan D, Munshi N, Treon SP, Maxwell CA, Pilarski L, Hideshima T, Hoffman RM, Anderson KC (2003) Fluorescence imaging of multiple myeloma cells in a clinically relevant SCID/NOD in vivo model: biologic and clinical implications. Cancer Res 63(20):6689–6696
Kim HS, Cho HR, Choi SH, Woo JS, Moon WK (2010) In vivo imaging of tumor transduced with bimodal lentiviral vector encoding human ferritin and green fluorescent protein on a 1.5 T clinical magnetic resonance scanner. Cancer Res 70(18):7315–7324. doi:10.1158/0008-5472.CAN-10-0241
Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, Brohee S, Salmon I, Dubois C, del Marmol V, Fuks F, Beck B, Blanpain C (2014) SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 511(7508):246–250. doi:10.1038/nature13305
Choy G, Choyke P, Libutti SK (2003) Current advances in molecular imaging: noninvasive in vivo bioluminescent and fluorescent optical imaging in cancer research. Mol Imaging 2(4):303–312
Kim YJ, Dubey P, Ray P, Gambhir SS, Witte ON (2004) Multimodality imaging of lymphocytic migration using lentiviral-based transduction of a tri-fusion reporter gene. Mol Imaging Biol: MIB: Off Publ Acad Mol Imaging 6(5):331–340. doi:10.1016/j.mibio.2004.06.009
Laxman B, Hall DE, Bhojani MS, Hamstra DA, Chenevert TL, Ross BD, Rehemtulla A (2002) Noninvasive real-time imaging of apoptosis. Proc Natl Acad Sci U S A 99(26):16551–16555. doi:10.1073/pnas.252644499
Min JJ, Nguyen VH, Kim HJ, Hong Y, Choy HE (2008) Quantitative bioluminescence imaging of tumor-targeting bacteria in living animals. Nat Protoc 3(4):629–636. doi:10.1038/nprot.2008.32
Rehemtulla A, Stegman LD, Cardozo SJ, Gupta S, Hall DE, Contag CH, Ross BD (2000) Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia 2(6):491–495
Wurdinger T, Badr C, Pike L, de Kleine R, Weissleder R, Breakefield XO, Tannous BA (2008) A secreted luciferase for ex vivo monitoring of in vivo processes. Nat Methods 5(2):171–173. doi:10.1038/nmeth.1177
Park JH, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ (2009) Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater 8(4):331–336. doi:10.1038/nmat2398
Vaijayanthimala V, Cheng PY, Yeh SH, Liu KK, Hsiao CH, Chao JI, Chang HC (2012) The long-term stability and biocompatibility of fluorescent nanodiamond as an in vivo contrast agent. Biomaterials 33(31):7794–7802. doi:10.1016/j.biomaterials.2012.06.084
Tse JW, Wiebe LI, Noujaim AA (1989) High specific activity [samarium-153] EDTA for imaging of experimental tumor models. J Nucl Med: Off Publ Soc Nucl Med 30(2):202–208
Boehm-Sturm P, Mengler L, Wecker S, Hoehn M, Kallur T (2011) In vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PLoS One 6(12), e29040. doi:10.1371/journal.pone.0029040
Miranda-Lorenzo I, Dorado J, Lonardo E, Alcala S, Serrano AG, Clausell-Tormos J, Cioffi M, Megias D, Zagorac S, Balic A, Hidalgo M, Erkan M, Kleeff J, Scarpa A, Sainz B Jr, Heeschen C (2014) Intracellular autofluorescence: a biomarker for epithelial cancer stem cells. Nat Methods 11(11):1161–1169. doi:10.1038/nmeth.3112
Hara M, Murakami T, Kobayashi E (2008) In vivo bioimaging using photogenic rats: fate of injected bone marrow-derived mesenchymal stromal cells. J Autoimmun 30(3):163–171. doi:10.1016/j.jaut.2007.12.007
Song F, Tian M, Zhang H (2014) Molecular imaging in stem cell therapy for spinal cord injury. Biomed Res Int 2014:759514. doi:10.1155/2014/759514
Contag CH, Bachmann MH (2002) Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng 4:235–260. doi:10.1146/annurev.bioeng.4.111901.093336
Contag CH, Ross BD (2002) It’s not just about anatomy: in vivo bioluminescence imaging as an eyepiece into biology. J Magn Reson Imaging : JMRI 16(4):378–387. doi:10.1002/jmri.10178
Hollingshead MG, Bonomi CA, Borgel SD, Carter JP, Shoemaker R, Melillo G, Sausville EA (2004) A potential role for imaging technology in anticancer efficacy evaluations. Eur J Cancer 40(6):890–898. doi:10.1016/j.ejca.2003.12.018
Au JT, Gonzalez L, Chen CH, Serganova I, Fong Y (2012) Bioluminescence imaging serves as a dynamic marker for guiding and assessing thermal treatment of cancer in a preclinical model. Ann Surg Oncol 19(9):3116–3122. doi:10.1245/s10434-012-2313-7
Schafer H, Schafer A, Kiderlen AF, Masihi KN, Burger R (1997) A highly sensitive cytotoxicity assay based on the release of reporter enzymes, from stably transfected cell lines. J Immunol Methods 204(1):89–98
Lehtinen J, Virta M, Lilius EM (2003) Fluoro-luminometric real-time measurement of bacterial viability and killing. J Microbiol Methods 55(1):173–186
Yang XY, Levi D, Ounissi-Benkalha H, Yu XY, Qu HQ, Polychronakos C, Du GH (2012) Screening for novel lead compounds increasing insulin expression in medullary thymic epithelial cells. Eur J Pharmacol 688(1–3):84–89. doi:10.1016/j.ejphar.2012.03.047
Chen H, Yao H, Huang L, Shen Q, Jia W, Xue J (2006) Expression of human factor IX gene in murine plasma through lentiviral vector-infected haematopoietic stem cells. Clin Exp Pharmacol Physiol 33(12):1196–1201. doi:10.1111/j.1440-1681.2006.04511.x
Poeschla EM, Wong-Staal F, Looney DJ (1998) Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med 4(3):354–357
Zhao J, Pettigrew GJ, Thomas J, Vandenberg JI, Delriviere L, Bolton EM, Carmichael A, Martin JL, Marber MS, Lever AM (2002) Lentiviral vectors for delivery of genes into neonatal and adult ventricular cardiac myocytes in vitro and in vivo. Basic Res Cardiol 97(5):348–358. doi:10.1007/s00395-002-0360-0
Chinnasamy N, Chinnasamy D, Toso JF, Lapointe R, Candotti F, Morgan RA, Hwu P (2000) Efficient gene transfer to human peripheral blood monocyte-derived dendritic cells using human immunodeficiency virus type 1-based lentiviral vectors. Hum Gene Ther 11(13):1901–1909. doi:10.1089/10430340050129512
Lee MS, Cohen B, Hand J, Nokes DJ (1999) A simplified and standardized neutralization enzyme immunoassay for the quantification of measles neutralizing antibody. J Virol Methods 78(1–2):209–217
Caceres G, Zhu XY, Jiao JA, Zankina R, Aller A, Andreotti P (2003) Imaging of luciferase and GFP-transfected human tumours in nude mice. Lumin: J Biol Chem Lumin 18(4):218–223. doi:10.1002/bio.729
Holic N, Seye AK, Majdoul S, Martin S, Merten OW, Galy A, Fenard D (2014) Influence of mildly acidic pH conditions on the production of lentiviral and retroviral vectors. Hum Gene Ther Clin Dev 25(3):178–185. doi:10.1089/humc.2014.027
Gilden DH, Wroblewska Z, Kindt V, Warren KG, Wolinsky JS (1978) Varicella-zoster virus infection of human brain cells and ganglion cells in tissue culture. Arch Virol 56(1–2):105–117
Eggermann J, Kliche S, Jarmy G, Hoffmann K, Mayr-Beyrle U, Debatin KM, Waltenberger J, Beltinger C (2003) Endothelial progenitor cell culture and differentiation in vitro: a methodological comparison using human umbilical cord blood. Cardiovasc Res 58(2):478–486
Saris SC, Spiess P, Lieberman DM, Lin S, Walbridge S, Oldfield EH (1992) Treatment of murine primary brain tumors with systemic interleukin-2 and tumor-infiltrating lymphocytes. J Neurosurg 76(3):513–519. doi:10.3171/jns.1992.76.3.0513
Clark AJ, Safaee M, Oh T, Ivan ME, Parimi V, Hashizume R, Ozawa T, James CD, Bloch O, Parsa AT (2014) Stable luciferase expression does not alter immunologic or in vivo growth properties of GL261 murine glioma cells. J Transl Med 12(1):345. doi:10.1186/s12967-014-0345-4
Acknowledgments
The FUGW vector was kindly provided by Dr. Yanmin Yang at Stanford University. This research was supported by the National Natural Science Foundation of China (81101089 and 81230034), by the Major State Basic Research Development Program of China (2013CB733800), and by Jiangsu Provincial Special Program for Medical Science (BL2013029).
Author Contributions
J. Ding conducted all experiments, integrated data, and edited figures; C. Wang performed the structural analysis and wrote the manuscript; P.C. Li, Z. Zhao, C. Qian, C.X. Wang and Y. Cai provided essential assistance; G.J. Teng directed this study, designed the research and gave key advices.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This manuscript has not been published or presented elsewhere in part or in entirety, and is not under consideration by another journal. All study participants provided informed consent, and the study design was approved by the appropriate ethics review boards.
Conflict of Interest
All authors discussed the results and approved the manuscript. There are no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Schematic representation of FUGW-Luc2-eGFP. Long terminal repeat, rev response element and woodchuck hepatitis virus posttranscriptional regulatory element were respectively abbreviated as LTR, RRE, and WRE. (GIF 174 kb)
Online Resource 2
Structural analysis of eGFP and Luc2. (A) Structure of eGFP in ribbon representation and hydrophobicity surface. (B) Superposition of Luc2 without cofactors (green) and Japanese Genji-botaru (Luciola cruciata) luciferase (magenta) in complex with cofactors AMP and oxyluciferin (yellow). (C) The cofactor-binding pocket in Luciola cruciata luciferase. (GIF 3605 kb)
Rights and permissions
About this article
Cite this article
Ding, J., Wang, C., Li, PC. et al. Dual-reporter Imaging and its Potential Application in Tracking Studies. J Fluoresc 26, 75–80 (2016). https://doi.org/10.1007/s10895-015-1673-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1673-3