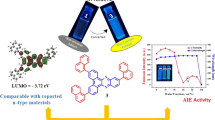
Abstract
The organic compounds with donor-π-bridge-acceptor type of architecture are of great interest for application as semiconductors. The synthesized compounds are obtained from 4-morpholino naphthalene-1,2-dione and 4-(4-(diethylamino) phenyl)naphthalene-1,2-dione and mono substituted ortho-phenylene diamines by condensation reaction. The donor groups are morpholinyl and N,N-diethylamino phenyl moieties, whereas acceptors are substituted phenazines. The synthesized molecules were characterized by spectral analysis.. The effect of the substitution has been studied on the basis of photophysical properties of the molecules. The halochromism behaviour of the molecule shows that at low to moderate acidity they respond differently with two types of donors. DFT computations were used in conjunction with NMR analysis to determine the ratio of the positional isomers.






Similar content being viewed by others
References
Li C-H, Kettle J, Horie M (2014) Cyclopentadithiophene–naphthalenediimide polymers; synthesis, characterisation, and n-type semiconducting properties in field-effect transistors and photovoltaic devices. Mater Chem Phys 144:519–528. doi:10.1016/j.matchemphys.2014.01.029
Zang X-F, Zhang T-L, Huang Z-S et al (2014) Impact of the position isomer of the linkage in the double D–A branch-based organic dyes on the photovoltaic performance. Dyes Pigments 104:89–96. doi:10.1016/j.dyepig.2013.12.028
Xu B, Wu X, Li H et al (2011) Selective detection of TNT and picric acid by conjugated polymer film sensors with donor—acceptor architecture. Macromolecules 44:5089–5092. doi:10.1021/ma201003f
Wang H, Lin J, Huang W, Wei W (2010) Fluorescence “turn-on” metal ion sensors based on switching of intramolecular charge transfer of donor—acceptor systems. Sensors Actuators B Chem 150:798–805. doi:10.1016/j.snb.2010.07.025
Liu X, Shu X, Zhou X et al (2010) Ultra-sensitive fluorescent sensor for Hg2+ based on a donor-acceptor-donor framework. J Phys Chem A 114:13370–13375. doi:10.1021/jp109304q
Basurto S, Riant O, Moreno D et al (2007) Colorimetric detection of Cu[II] cation and acetate, benzoate, and cyanide anions by cooperative receptor binding in new alpha, alpha’-bis-substituted donor-acceptor ferrocene sensors. J Org Chem 72:4673–4688. doi:10.1021/jo0702589
Chang YJ, Chow TJ (2011) Highly efficient red fluorescent dyes for organic light-emitting diodes. J Mater Chem 21:3091–3099. doi:10.1039/c0jm03109g
Chen C-T (2004) Evolution of red organic light-emitting diodes: materials and devices. Chem Mater 16:4389–4400. doi:10.1021/cm049679m
Kim S-J, Zhang Y, Zuniga C et al (2011) Efficient green OLED devices with an emissive layer comprised of phosphor-doped carbazole/bis-oxadiazole side-chain polymer blends. Org Electron 12:492–496
Ni YR, Su HQ, Huang W et al (2013) A spiro [fluorene-9, 9′-xanthene]-based host material for efficient green and blue phosphorescent OLED. Appl Mech Mater 331:503–507
Sun Q, Li D, Dong G et al (2013) Improved organic optocouplers based on a deep blue fluorescent OLED and an optimized bilayer heterojunction photosensor. Sensors Actuators B Chem 188:879–885
Kessler F, Watanabe Y, Sasabe H et al (2013) High-performance pure blue phosphorescent OLED using a novel bis-heteroleptic iridium(iii) complex with fluorinated bipyridyl ligands. J Mater Chem C 1:1070. doi:10.1039/c2tc00836j
Wang L, Shi Y, Zhao Y et al (2014) “Push–pull” 1,8-naphthalic anhydride with multiple triphenylamine groups as electron donor. J Mol Struct 1056–1057:339–346. doi:10.1016/j.molstruc.2013.10.004
Sudyoadsuk T, Pansay S, Morada S et al (2013) Synthesis and characterization of D-D-π-A-type organic dyes bearing carbazole-carbazole as a donor moiety (D-D) for efficient dye-sensitized solar cells. Eur J Org Chem 2013:5051–5063. doi:10.1002/ejoc.201300373
Hua Y, Chang S, Wang H et al (2013) New phenothiazine-based dyes for efficient dye-sensitized solar cells: positioning effect of a donor group on the cell performance. J Power Sources 243:253–259. doi:10.1016/j.jpowsour.2013.05.157
Jamorski CJ, Casida ME (2004) Time-dependent density-functional theory investigation of the fluorescence behavior as a function of alkyl chain size for the 4-( N, N -dimethylamino)benzonitrile-like donor−acceptor systems 4-( N, N -diethylamino)benzonitrile and 4-( N, N -diisopropyl). J Phys Chem B 108:7132–7141. doi:10.1021/jp0307699
Chang DM, Kwon DY, Kim YS (2013) Heteroleptic dual acceptor organic dyes with rhodanine-3-acetic acid and cyanoacrylic acid. Mol Cryst Liq Cryst 585:100–106. doi:10.1080/15421406.2013.850933
Zhu L, Yang H, Zhong C, Li CM (2012) Modified triphenylamine-dicyanovinyl-based donor-acceptor dyes with enhanced power conversion efficiency of p-type dye-sensitized solar cells. Chem Asian J 7:2791–2795. doi:10.1002/asia.201200402
Agneeswari R, Tamilavan V, Song M et al (2013) Synthesis of polymers containing 1,2,4-oxadiazole as an electron-acceptor moiety in their main chain and their solar cell applications. J Polym Sci Part A Polym Chem 51:2131–2141. doi:10.1002/pola.26605
Lee W, Seng JY, Hong J-I (2013) Metal-free organic dyes with benzothiadiazole as an internal acceptor for dye-sensitized solar cells. Tetrahedron 69:9175–9182. doi:10.1016/j.tet.2013.08.075
Xu Z, Wang M, Zhao J et al (2014) Donor–acceptor type neutral green polymers containing 2,3-di(5-methylfuran-2-yl) quinoxaline acceptor and different thiophene donors. Electrochim Acta 125:241–249. doi:10.1016/j.electacta.2013.12.097
Becerril HA, Miyaki N, Tang ML et al (2009) Transistor and solar cell performance of donor–acceptor low bandgap copolymers bearing an acenaphtho[1,2-b]thieno[3,4-e]pyrazine (ACTP) motif. J Mater Chem 19:591. doi:10.1039/b819210c
Li Y, Meng B, Tong H et al (2014) A chlorinated phenazine-based donor–acceptor copolymer with enhanced photovoltaic performance. Polym Chem 5:1848. doi:10.1039/c3py01436c
Rurack K, Bricks JL, Reck G et al (2000) Chalcone-analogue dyes emitting in the near-infrared (NIR): influence of donor-acceptor substitution and cation complexation on their spectroscopic properties and X-ray structure. J Phys Chem A 104:3087–3109
Magde D, Wong R, Seybold PG (2002) Fluorescence quantum yields and their relation to lifetimes of rhodamine 6G and fluorescein in nine solvents: improved absolute standards for quantum yields. Photochem Photobiol 75:327. doi:10.1562/0031-8655(2002)075<0327:FQYATR>2.0.CO;2
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138. doi:10.1103/PhysRev.140.A1133
Becke AD (1993) Density-functional thermochemistry.III. The role of exact exchange. J Chem Phys 98:5648. doi:10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3093. doi:10.1021/cr9904009
Ditchfield R (1971) Self-consistent molecular-orbital methods. IX. an extended gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54:724. doi:10.1063/1.1674902
Krishnan R, Schlegel HB, Pople JA (1980) Derivative studies in configuration–interaction theory. J Chem Phys 72:4654. doi:10.1063/1.439708
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09, revision C.01. Gaussian 09, revis. B.01. Gaussian, Inc, Wallingford
Martin EL, Fieser LF (1941) 1,2-naphthoquinone-4-sulfonate, ammonium and potassium. Org Synth 21:91. doi:10.15227/orgsyn.021.0091
Van Gernert B, Knowles DB (1996) Photochromic Naphthopyran Compounds :1–10
Rickwood M, Marsden SD, Askew VE (1995) Photochromic Spiroxazine Compounds :1–8
Lakowicz JR (2007) Principles of fluorescence spectroscopy. Springer Science & Business Media, New York
Rtishchev NI, Samoilov DV, Martynova VP, El’tsov AV (2001) Luminescence properties of nitro derivatives of fluorescein. Russ J Gen Chem 71:1467–1478. doi:10.1023/A:1013974507390
Samori S, Tojo S, Fujitsuka M et al (2007) Donor-acceptor-substituted tetrakis(phenylethynyl)benzenes as emissive molecules during pulse radiolysis in benzene. J Org Chem 72:2785–2793. doi:10.1021/jo062326h
Beinhoff M, Weigel W, Jurczok M et al (2001) Synthesis and spectroscopic properties of arene-substituted pyrene derivatives as model compounds for fluorescent polarity probes. Eur J Org Chem 2001:3819–3829. doi:10.1002/1099-0690(200110)2001:20<3819::AID-EJOC3819>3.0.CO;2-W
Lippert E (1957) Spektroskopische Bestimmung des Dipolmomentes aromatischer Verbindungen im ersten angeregten Singulettzustand. Z Elektrochem Ber Bunsenges Phys Chem 61:962–975. doi:10.1002/bbpc.19570610819
Valeur B (2001) Mol Fluorescence. doi:10.1002/3527600248
Singh P, Baheti A, Thomas KRJ (2011) Synthesis and optical properties of acidochromic amine-substituted benzo[a]phenazines. J Org Chem 76:6134–6145
Achelle S, Barsella A, Baudequin C et al (2012) Synthesis and photophysical investigation of a series of push–pull arylvinyldiazine chromophores. J Org Chem 77:4087–4096. doi:10.1021/jo3004919
Achelle S, Rodríguez-López J, Robin-le Guen F (2014) Synthesis and photophysical studies of a series of quinazoline chromophores. J Org Chem 79:7564–7571. doi:10.1021/jo501305h
Shailajha S, Rajesh Kannan U, Sheik Abdul Kadhar SP, Isac Paulraj E (2014) Molecular structure, vibrational spectra and (13)C and (1)H NMR spectral analysis of 1-methylnaphthalene by ab initio HF and DFT methods. Spectrochim Acta A Mol Biomol Spectrosc 133:720–729. doi:10.1016/j.saa.2014.06.006
Gupta R, Chaudhary RP (2014) Studies on orientation of cyclization in thiazolo-quinazoline heterocyclic system through NMR, DFT, and X-ray diffraction. J Heterocycl Chem. doi:10.1002/jhet.2098
Alkorta I, Elguero J (1998) Ab initio hybrid DFT–GIAO calculations of the shielding produced by carbon–carbon bonds and aromatic rings in 1H NMR spectroscopy. New J Chem 22:381–385. doi:10.1039/a708743h
Olah GA, Rasul G, Heiliger L, Prakash GKS (1996) Preparation, NMR spectroscopic, and ab initio /DFT/GIAO-MP2 studies of halomethyl cations 1. J Am Chem Soc 118:3580–3583. doi:10.1021/ja9538905
Acknowledgments
Abhinav Tathe is thankful to University Grants Commission, New Delhi for JRF and SRF fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 818 kb)
Rights and permissions
About this article
Cite this article
Tathe, A.B., Sekar, N. Novel Fluorescent Phenazines : Synthesis, Characterization, Photophysical Properties and DFT Calculations. J Fluoresc 25, 1403–1415 (2015). https://doi.org/10.1007/s10895-015-1631-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1631-0