Abstract
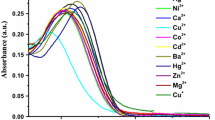
A new fluorescent probe based on a bis-benzimidazole diamide N 2,N 2′-bis[(1-ethyl-benzimidazol-2-yl)methyl]biphenyl-2,2′-dicarboxamide ligand L 1 with a biphenyl spacer group and a Copper(II) trinuclear metallacycle has been synthesized and characterized by X-ray single crystallography, elemental and spectral (FT-IR, 1H & 13C NMR, UV-Visible) analysis. The fluorescence spectra of L 1 in MeOH show an emission band centered at 300 nm. This band arises due to benzimidazolyl moiety in the ligating system. The diamide L 1 in the presence of Cu2+ show the simultaneous ‘quenching’ of (300 nm) and ‘enhancement’ of (375 nm) emission band. Similar fluorescence behavior was found in water–methanol mixture (9:1). The new emission band at 375 nm is attributed to intra ligand π–π* transition of the biphenyl moiety. L 1 exhibited high selectivity and sensitivity towards Cu2+ in both the medium over other common metal ions like Ni2+, Co2+, Mn2+, Mg2+, Zn2+, Pb2+ and Hg2+. The binding constant with Cu2+ was calculated by the Benesi-Hildebrand equation. Selective “off-on-off” behavior of L 1 in methanol has also been studied. The fluorescent intensity of 375 nm bands in L 1 enhances (turns-on) upon addition of Cu2+ and quenches (turn-off) upon addition of Na2-EDTA.









Similar content being viewed by others
References
Lovstad RA (2004) A kinetic study on the distribution of Cu(II)-ions between albumin and transferrin. BioMetals 17:111–113
Que EL, Domaille DW, Chang CJ (2008) Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev 108:1517–1549
Gaggelli E, Kozlowski H, Valensin D, Valensin G (2006) Copper homeostasis and neurodegenerative disorders (Alzheimer’s, Prion, and Parkinson’s Diseases and Amyotrophic Lateral Sclerosis). Chem Rev 106:1995–2044
Jung HS, Park M, Han DY, Kim E, Lee C, Ham S, Kim JS (2009) Cu2+ ion-induced self-assembly of pyrenylquinoline with a pyrenyl excimer formation. Org Lett 11:3378–3381
Zheng Y, Gattas-Asfura KM, Konka V, Leblanc RM (2002) A dansylated peptide for the selective detection of copper ions. Chem Commun 2350–2351
Jung et al (2009) Coumarin-derived Cu2+ -selective fluorescence sensor: synthesis, mechanisms, and applications in living cells. J Am Chem Soc 131:2008–2012
Weng YQ, Yue F, Zhong YR, Ye BH (2007) A Copper(II) ion-selective on-off-type fluoroionophore based on zinc porphyrin–dipyridylamino. Inorg Chem 46:7749–7755
Xu Z, Yoon J, Spring DR (2010) Fluorescence chemosensors for Zn2+. Chem Soc Rev 39:1996–2006
Callan JF, De Silva AP, Magri DC (2005) Luminescent sensors and switches in the early 21st century. Tetrahedron 61:8551–8588
Li J et al (2011) A novel rhodamine-benzimidazole conjugate as a highly selective turn-on fluorescent probe for Fe3+. J Fluoresc 21:2005–2013
Khatua S, Choi SH, Lee J, Huh JO, Do Y, Churchill DJ (2009) Highly selective fluorescence detection of Cu2+ in water by chiral dimeric Zn2+ complexes through direct displacement. Inorg Chem 48:1799–1801
Royzen M, Dai Z, Canary JW (2005) Ratometric displacement approach to Cu(II) sensing by fluorescence. J Am Chem Soc 127:1612–1613
Wolf C, Mei XF, Rokadia HK (2004) Selective detection of Fe(III) ions in aqueous solution with a 1,8-diacridylnaphthalene-derived fluorosensor. Tetrahedron Lett 45:7867–7871
Ballesteros E et al (2009) A new selective chromogenic and turn-on fluorogenic probe for copper(II) in water–acetonitrile 1:1 solution. Org Lett 11:1269–1272
Shin DH, Ko YG, Choi US, Kim WN (2006) Bowing effect with fluorescence: a unique chemosensor for the silver ion. Ind Eng Chem Res 45:656–662
Formica M, Fusi V, Giorgi L, Micheloni M (2012) New fluorescent chemosensors for metal ions in solution. Coord Chem Rev 256:170–192
De Silva SA, Zavaleta AD, Baron E (1997) A fluorescent photoinduced electron transfer sensor for cations with an off-on-off proton switch. Tetrahedron Lett 38:2237–2240
De Silva SA et al (2005) A fluorescent “off-on-off” proton switch derived from natural products and further studies of first-generation fluorescent photoinduced electron transfer (PET) systems. J Mater Chem 15:2791–2795
De Silva SA et al (2002) A fluorescent “off-on-off” proton switch with an overriding ‘enable-disable’ sodium ion switch. Chem Commun 1360–1361
Callan JF, De Silva AP, McClenaghanb ND (2004) Switching between molecular switch types by module rearrangement: Ca2+-enabled, H+-driven ‘off-on-off’, H+-driven YES and PASS 0 as well as H+, Ca2+-driven AND logic operations. Chem Commun 2048–2049
Goswami P, Das DK (2011) Significant effect of surfactant micelles on pH dependent fluorescent off-on-off behavior of Salicylaldehyde-2,4-Dinitrophenylhydrazone. J Luminescence 131:760–763
Bandyopadhyay P, Ghosh AK (2009) pH controlled “off-on-off” switch based on Cu2+-mediated pyrene fluorescence in a PAA–SDS micelle aggregated supramolecular system. J Phys Chem B 113:13462–13464
Pais VF et al (2011) Off-on-off fluorescence switch with T-Latch function. Org Lett 13:5572–5575
Fabbrizzi L, Gatti F, Pallavicini P, Parodi L (1998) An “off-on-off” fluorescent sensor for pH based on ligand–proton and ligand–metal–proton interactions. New J Chem 1403–1407
Zhang HG et al (2011) Off-on-off luminescent switching of a dye containing imidazo[4, 5-f][1, 10]phenanthroline. Chin Chem Lett 22:647–650
Ravikumar I, Ghosh P (2011) Zn(II) and PPi selective fluorescence off-on-off functionality of a chemosensor in physiological conditions. Inorg Chem 50:4229–4231
Pandey R et al (2011) Fluorescent zinc(II) complex exhibiting “on-off-on” switching toward Cu2+ and Ag+ ions. Inorg Chem 50:3189–3197
Barceloux DG, Barceloux D (1999) Copper. J Toxicol Clin Toxicol 37:217–230
Zhang XB, Peng J, He CL, Shen GL, Yu RQ (2006) A highly selective fluorescent sensor for Cu2+ based on 2-(2′-hydroxyphenyl)benzoxazole in a poly(vinyl chloride) matrix. Anal Chim Acta 567:189–195
Sarkar B (1981) In Metal ions in biological systems. Siegel H, Siegel A (eds.) Marcel Dekker, New York 12:233
Georgopoulos PG, Roy A, Yonone-Lioy MJ, Opiekun RE, Lioy PJ (2001) Environmental copper: its dynamics and human exposure issues. J Toxicol Environ Health B 4:341–394
Tak WT, Yoon SC (2001) Clinical significance of blood level of zinc and copper in chronic renal failure patients. KSN 20:863–871
Zheng Y et al (2003) Development of fluorescent film sensors for the detection of divalent copper. J Am Chem Soc 125:2680–2686
Zheng Y et al (2001) A new fluorescent chemosensor for copper ions based on tripeptide Glycyl–Histidyl–Lysine (GHK). Org Lett 3:3277–3280
Zheng Y et al (2003) Design of a membrane fluorescent sensor based on photo-cross-linked PEG hydrogel. J Phys Chem B 107:483–488
Goswami S, Chakrabarty R (2009) Fluorescence sensing of Cu2+ within a pseudo 18-crown-6 cavity. Tetrahedron Lett 50:5910–5913
Liu Z, Yang Z, Li T, Wang B, Li Y, Qin D, Wang M, Yan M (2011) An effective Cu(II) quenching fluorescence sensor in aqueous solution and 1D chain coordination polymer framework. Dalton Trans 40:9370–9373
Costero AM, Gil S, Sanchis J, Peransi S, Sanz V, Williams JAG (2004) Conformationally regulated fluorescent sensors. Study of the selectivity in Zn2+ versus Cd2+ sensing. Tetrahedron 60:6327–6334
Cescon LA, Day AR (1962) Preparation of some benzimidazolylamino acids. Reactions of amino acids with o-phenylenediamines. J Org Chem 27:581–586
Mahiya K, Mathur P (2013) Morphology dependent oxidation of aromatic alcohols by new symmetrical copper (II) metallatriangles formed by self-assembly of a shared bis -benzimidazolyl diamide ligand. Inorganica chimica Acta. doi:10.1016/j.ica.2012.12.037
Barnes DJ, Chapman RL, Vagg RS, Watton EC (1978) Synthesis of novel bis(amides) by means of triphenyl phosphite intermediates. J Chem Eng Data 23:349–350
Sheldrick GM (1997) SHELXS97 and SHELXL97; program for crystal structure solution and refinement. University of Gottingen, Germany
Farrugia LJ (1999) WinGX suite for small-molecule single-crystal crystallography. J Appl Crystallogr 32:837–838
Kumar A, Sinha HK, Dogra SK (1989) Electronic spectrum of bibenzimidazole homologue: effects of solvents and acid concentration. Can J Chem 67:1200–1205
Huang HW et al (1996) Fluorescence study on intermolecular interactions between mesogenic biphenyl moieties of a thermotropic liquid-crystalline polyester (PB-10). Macromolecules 29:3485–3490
Guo F, Xu J, Zhang X, Zhu B (2010) Hydrothermal synthesis, crystal structures and photoluminescent properties of four cadmium(II) coordination polymers derived from diphenic acid and auxiliary ligands. Inorg Chim Acta 363:3790–3797
Zhang LY et al (2003) Helical ribbons of Cadmium(II) and Zinc(II) dicarboxylates with bipyridyl-like chelates–syntheses, crystal structures and photoluminescence. Eur J Inorg Chem 2003:2965–2971
Chen W et al (2003) Photoluminescent metal–organic polymer constructed from trimetallic clusters and mixed carboxylates. Inorg Chem 42:944–946
Yam VWW, Lo KKW (1999) Luminescent polynuclear d10 metal complexes. Chem Soc Rev 28:323–334
Benesi HA, Hildebrand JH (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707
Barra M, Bohne C, Scaiano JC (1990) Effect of cyclodextrin complexation on the photochemistry of xanthone. Absolute measurement of the kinetics for triplet-state exit. J Am Chem Soc 112:8075–8079
Xie G et al. (2012) A highly selective fluorescent and colorimetric chemosensor for ZnII and its application in cell imaging. Eur J Inorg Chem 327
Ghosh K, Sen T, Patra A (2010) Binding induced destruction of an excimer in anthracene-linked benzimidazole diamide: a case toward the selective detection of organic sulfonic acids and metal ions. New J Chem 34:1387–1393
Huo F-J, Su Y-Q, Su J, Yang Y-T, Yin C-X, Chao J-B (2010) Chromene “lock”, thiol “key”, and Mercury(II) ion “hand”: a single molecular machine recognition system. Org Lett 12:4756–4759
Monzani E et al (1998) Tyrosinase models. synthesis, structure, catechol oxidase activity, and phenol monooxygenase activity of a dinuclear copper complex derived from a triamino pentabenzimidazole ligand. Inorg Chem 37:553–562
Tehlan S, Hundal MS, Mathur P (2004) Copper(II) complexes of N-Octylated Bis(benzimidazole) diamide ligands and their peroxide-dependent oxidation of aryl alcohols. Inorg Chem 43:6589–6595
Addison AW, Rao TN, Reedijk J, Rijn JV, Verschoor GC (1984) Synthesis, structure, and spectroscopic properties of Copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of Aqua[l,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) Perchlorate. J Chem Soc Dalton Trans 1349–1356
Spek AL (2003) Single-crystal structure validation with the program. PLATON J Appl Crystallogr 36:7–13
Acknowledgments
The authors are grateful to University of Delhi, Delhi for a special grant. We are thankful to USIC-CIF, University of Delhi, Delhi, India for NMR and Single Crystal X-ray data. One of the authors is thankful to UGC for providing senior research fellowship (SRF).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1303 kb)
Rights and permissions
About this article
Cite this article
Mahiya, K., Mathur, P. Bis-Benzimidazolyl Diamide Based Fluorescent Probe for Copper(II): Synthesis, Structural and Fluorescence Studies. J Fluoresc 23, 767–776 (2013). https://doi.org/10.1007/s10895-013-1182-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-013-1182-1