Abstract
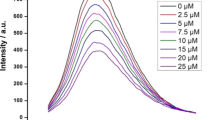
Cystatins are thiol proteinase inhibitors ubiquitously present in mammalian body and serve various important physiological functions. In the present study, we examined the effects of acid denaturation on newly identified thiol protease inhibitors from the lungs of Capra hircus (Goat) with a focus on protein conformational changes and amyloid fibril formation. Acid denaturation as studied by CD (Circular Dichroism) and fluorescence spectroscopy showed that purified inhibitor named GLC (Goat Lung Cystatin) populates three partly unfolded species, a native like state at pH 3.0, a partly unfolded intermediate at pH2.0, and unstructured unfolded state at pH 1.0, from each of which amyloid like fibrils grow as assessed by thioflavin T (ThT) spectroscopy. The result showed, native like structure formed at pH 3.0 is more responsive towards amyloid formation when compare to other conformation of proteins. Morphology of the protein species incubated for amyloid process was observed using transmission electron microscopy (TEM). Moreover, anti-fibrillogenic effects of curcumin and quercetin were analysed using ThT binding assay. Curcumin and quercetin produced a concentration dependent decline inThT fluorescence suggesting deaggregation of the fibrils. When added prior to amyloid fibril initiation 50 μM curcumin inhibited amyloid aggregation. However, more quercetin is needed to prevent the same extent of fibrillation. Implications for therapeutics in view of polyphenols as essential nutrients are suggested in lung diseases.





Similar content being viewed by others
References
Schlehe JS, Lutz AK, Pilsl A et al (2008) Aberrant folding of pathogenic Parkin mutants: aggregation versus degradation. J Biol Chem 283:13771–13779
Luheshi LM, Dobson CM (2009) Bridging the gap: from protein misfolding to protein misfolding diseases. FEBS Lett 583:2581–2586
Winklhofer KF, Tatzelt J, Haass C (2008) The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J 27:336–349
Pedersen CB, Bross P, Winter VS et al (2003) Misfolding, degradation, and aggregation of variant proteins. The molecular pathogenesis of short chain acyl-CoA dehydrogenase (SCAD) deficiency. J Biol Chem 278:47449–47458
Pedersen CB, Kolvraa S, Kolvraa A et al (2008) The ACADS gene variation spectrum in 114 patients with short-chain acyl-CoA dehydrogenase (SCAD) deficiency is dominated by missense variations leading to protein misfolding at the cellular level. Hum Genet 124:43–56
Dobson CM (1999) Protein misfolding, evolution and disease. Trends Biochem Sci 24:329–332
Rochet JC, Lansbury PT (2000) Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol 10:60–68
Uversky VN, Fink AL (2004) Conformational constraints for amyloid fibrillation: the importance of being unfolded. Biochim Biophys Acta 1698:131–153
Chiti F, Taddei N, Bucciantini M, White P, Rampovi G, Dobson CM (2000) Mutational analysis of the propensity for amyloid formation by a globular protein. EMBO J 19:1441–1449
Wilkins DK, Dobson CM, Gross M (2000) Biophysical studies of the development of amyloid fibrils from a peptide fragment of cold shock protein B. Eur J Biochem 267:2609–2616
Krebs MR, Wilkins DK, Chung EW, Pitkeathy MC, Chamberlin AK, Zurdo J, Robinson CV, Dobson CM (2000) Formation and seeding of amyloid fibrils from wild-type hen lysozyme and a peptide fragment from the beta-domain. J Mol Biol 300:541–549
Ramirez-Alvarado M, Merkel JS, Regan LA (2000) A systematic exploration of the influence of the protein stability on amyloid fibril formation in vitro. PNAS 97:8974–8984
Zurdo J, Guijarro JI, Jimenez JL, Saibil HR, Dobson CM (2001) Dependence on solution conditions of aggregation and amyloid formation by an SH3 domain. J Mol Biol 311:325–340
Pertinhez TA, Bouchard M, Tomlinson EJ, Wain R, Ferguson SJ, Dobson CM (2001) Amyloid fibril formation by a helical cytochrome. FEBS Lett 495:184–186
Fandrich M, Flectcher MA, Dobson CM (2001) Amyloid fibrils from muscle myoglobin. Nature 410:165–166
Priyadarshini M, Bano B (2012) Conformational changes during amyloid fibril formation of pancreatic thiol proteinase inhibitor: effect of copper and zinc. Mol Biol Rep 39:2945–2955
Rashid F, Sharma S, Baig MA, Bano B (2006) Molten globule state of human placental cystatin (HPC) at low pH conditions and the effects of trifluoroethanol (TFE) and methanol. Biochem Cell Biol 84:126–134
Ahmad B, Khan RH (2006) Studies on the acid unfolded and molten globule states of catalytically active stem bromelain: a comparison with catalytically inactive form. J Biochem 140:501–508
Zerovnik E, Jerala R, Kroon-Zitko L (1992) Intermediates in denaturation of a small globular protein, recombinant human stefin B. Biol Chem 267:9041–9046
Khurana R, Ionescu-Zanetti C, Pope MLJ, Nielson L, Ramirez-Al Varado M, Regan L, Fink AL, Carter SA (2003) A general model for amyloid fibril assembly based on morphological studies using atomic force microscopy. Biophy J 85:1135–1144
Ahmad A et al (2004) The cellular prion protein PrPc is expressed in human enterocytes in cell-cell junctional domains. Biol Chem 279:1499–15013
Williams AD, Portelius E, Kheterpal I, Guo JT, Cook KD, Wetzel YX (2004) Mapping abeta amyloid fibril secondary structure using scanning proline mutagenesis. J Mol Biol 335:833–842
Priyadarshini M, Bano B (2010) Cystatin like thiol proteinase inhibitor from pancreas of Capra hircus: purification and detailed biochemical characterization. Amino Acids 38:1001–1010
Turk V, Stoka V, Turk D (2008) Cystatins: biochemical and structural properties, and medical relevance. Frontier Bioscience 13:5406–5420
Skerget K, Vilfan A, Pompe-Novak M et al (2009) The mechanism of amyloid-fibril formation by stefin B: temperature and protein concentration dependence of the rates. Proteins 74:425–436
Khan MS, Bano B (2009) Purification, characterization and kinetics of thiol protease inhibitor from goat (Capra hircus) lung. Biochemistry (Moscow Russ Fed) 74:781–788
Tyagi S, Singh G, Sharma A (2010) Effect of garlic on cardiovascular disorders: a review. J Pharmac Sci Rev Res 3:53–55
Cheng B, Liu X, Gong H, Huang L, Chen H, Zhang X, Li C, Yang M, Ma B, Jiao L, Zheng L, Huang K (2011) Coffee components inhibit amyloid formation of human islet amyloid polypeptide in vitro: possible link between coffee consumption and diabetes mellitus. J Agric Food Chem 59:13147–13155
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mulqueen PM, Kronamn MJ (1982) Binding of naphthalene dyes to the N and A conformers of bovine alpha-lactalbumin. Arch Biochem Biophys 215:28–39
Wang SSS, Liu KN, Han TC (2010) Amyloid fibrillation and cytotoxicity of insulin are inhibited by the amphiphilic surfactants. Biochim Biophys Acta 1802:519–530
Zerovnik E, Skarabot M, Skerget K et al (2007) Amyloid fibril formation by human stefin B: influence of pH and TFE on fibril growth and morphology. Amyloid 14:237–247
LeVine H (1999) Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol 309:274–284
Ahmad A, Uversky VN, Hong D et al (2005) Early events in the fibrillation of monomeric insulin. J Biol Chem 280:42669–42675
He Y, Zhou H, Tang H et al (2006) Deficiency of disulfide bonds facilitating fibrillogenesis of endostatin. J Biol Chem 281:1048–1057
Jenko S, Skaraboot M et al (2004) Different propensity to form amyloid fibrils by two homologous proteins-Human stefins A and B: searching for an explanation. Proteins Struct Funct Bioinf 55:417–425
Rabzelj S, Turk V, Zerovnik E (2005) In vitro study of stability and amyloid-fibril formation of two mutants of human stefin B (cystatin B) occurring in patients with EPM1. Protein Sci 14:2713–2722
Holm NK, Jespersen SK, Thomassen LV et al (2007) Aggregation and fibrillation of bovine serum albumin. Biochim Biophys Acta 1774:1128–1138
Wang SSS, Liu KN, Lee WH (2009) Effect of curcumin on the amyloid fibrillogenesis of hen egg-white lysozyme. Biophys Chem 144:78–87
Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM (2005) Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280:5892–5901
Davinelli S, Sapere N, Zella D, Bracale R, Intrieri M, Scapagnini G (2012) Pleiotropic protective effects of phytochemicals in Alzheimer’s disease. Oxid Med Cell Longev. doi:10.1155/2012/386527
Acknowledgements
The authors extend their appreciation to the deanship of scientific research at KSU for funding the work through the research group project No- RGP-VPP-151.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, M.S., Al-Senaidy, A.M., Priyadarshini, M. et al. Different Conformation of Thiol Protease Inhibitor During Amyloid Formation: Inhibition by Curcumin and Quercetin. J Fluoresc 23, 451–457 (2013). https://doi.org/10.1007/s10895-013-1158-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-013-1158-1