Abstract
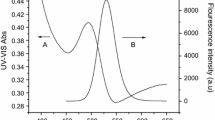
This paper describes the development of a simplified and rapid method for the aqueous synthesis of quantum dots (QDs) with CdTe cores and gradient CdS external shells (CdTe/CdS QDs) aided by microwave irradiation. Several synthesis parameters, such as molar ratio of reagents, pH, reaction temperature, and reaction time, were studied in details. Under the optimized conditions, highly effective CdTe/CdS QDs could be synthesized in aqueous phase in only 15 min. In order to improve the biocompatibility of the CdTe/CdS QDs, these QDs were then interacted with carboxymethyl chitosan (CMC) so as they could be used as fluorescent probes in the aqueous phase. With the incorporation of CMC, the stability of modified QDs was found to have improved significantly (from 4 months to more than 10 months at room temperature). The photoluminescence quantum yield (PLQY) of the modified QDs could reach 75%, other parameters include a full width at half maximum of the emission (FWHM) spectrum as 40 ~ 60 nm, and an average size, estimated from electron microscopic images, as 3.5 nm. As fluorescent probes, these modified QDs were successfully used for imaging live Madin–Darby canine kidney (MDCK) cells, in which the preliminary results indicated that these modified QDs demonstrated good biocompatibility and showed promising applications for bio-labeling and imaging.









Similar content being viewed by others
References
Vossmeyer T, Katsikas L, Giersig M, Popovic IG, Diesner K, Chemseddine A, Eychmueller A, Weller H (1994) CdS nanoclusters: synthesis, characterization, size dependent oscillator strength, temperature shift of the excitonic transition energy, and reversible absorbance shift. J Phys Chem 98(31):7665–7673
Rogach AL, Kornowski A, Gao M, Eychmüller A, Weller H (1999) Synthesis and characterization of a size series of extremely small thiol-stabilized CdSe nanocrystals. J Phys Chem B 103(16):3065–3069
Zhang H, Wang LP, Xiong HM, Hu LH, Yang B, Li W (2003) Hydrothermal synthesis for high-quality CdTe nanocrystals. Adv Mater 15(20):1712–1715
Gaponik N, Talapin DV, Rogach AL, Hoppe K, Shevchenko EV, Kornowski A, Eychmüller A, Weller H (2002) Thiol-capping of CdTe nanocrystals: an alternative to organometallic synthetic routes. J Phys Chem B 106:7177–7185
Rogach AL, Katsikas L, Kornowski A, Su D, Eychmüller A, Weller H (1996) Synthesis and characterization of thiol-Stabilized CdTe nanocrystals. Ber Bunsenges Phys Chem 100:1772–1778
Li M, Ge Y, Chen Q, Xu S, Wang N, Zhang X (2007) Hydrothermal synthesis of highly luminescent CdTe quantum dots by adjusting precursors’ concentration and their conjunction withBSA as biological fluorescent probes. Talanta 72:89–94
Kurth DG, Lehmann P, Lesser C (2000) Engineering the surface chemical properties of semiconductor nanoparticles: surfactant-encapsulated CdTe-clusters. Chem Commun 11:949–950
Wang C, Gao X, Su XG (2010) In vitro and in vivo imaging with quantum dots. Anal Bioanal Chem 397:1397–1415
Grisaru H, Palchik O, Gedanken A, Slifkin MA, Weiss AM, Palchik V (2003) Microwave-assisted polyol synthesis of CuInTe2 and CuInSe2 nanoparticles. Inorg Chem 42:7148–7155
Qian H, Li L, Ren J (2005) One-step and rapid synthesis of high quality alloyed quantum dots (CdSe/CdS) in aqueous phase by microwave irradiation with controllable temperature. Mater Res Bull 40:1726–1736
Song L, Duan J, Zhan J (2010) One-pot Microwave assisted synthesis of homogeneously alloyed CdSexTe1−x nanocrystals with tunable photoluminescence. Mater Lett 64:1843–1845
Liao XH, Chen NY, Xu S, Yang SB, Zhu JJ (2003) A microwave assisted heating method for the preparation of copper sulfide nanorods. J Cryst Growth 252:593–598
Grisaru H, Palchik O, Gedanken A, Slifkin MA, Weiss AM, Palchik V (2003) Microwave-assisted polyol synthesis of CuInTe2 and CuInSe2 nanoparticles. Inorg Chem 42:7148–7155
Palchik O, Zhu JJ, Gedanken A (2000) Microwave assisted preparation of binary oxide nanoparticles. J Mater Chem 10:1251–1254
Zhu JJ, Palchik O, Chen SG, Gedanken A (2000) Microwave assisted preparation of CdSe, PbSe, and Cu2-xSe nanoparticles. J Phys Chem B 104:7344–7347
Gao XH, Cui YY, Levenson RM, Chung LWK, Nie SM (2004) In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol 22:969–976
Alivisatos P (2004) The use of nanocrystals in biological detection. Nat Biotechnol 22:47–52
Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S (2005) Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307:538–544
Howarth M, Takao K, Hayashi Y, Ting AY (2005) Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc Natl Acad Sci 102:7583–7588
Lovric J, Bazzi HS, Cuie Y, Fortin GRA, Winnik FM, Maysinger D (2005) Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J Mol Med 83:377–385
Lovric J, Cho SJ, Winnik FM, Maysinger D (2005) Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem Biol 12:1227–1234
Muzzarelli R, Baldassarre V, Conti F, Ferrara P, Biagini G, Gazzanelli G, Vasi V (1988) Biological activity of chitosan: ultrastructural study. Biomaterials 9:247–252
Jigar MJ, Sinha VK (2007) Ceric ammonium nitrate induced grafting of polyacrylamide onto carboxymethyl chitosan. Carbohyd Polym 67:427–435
Wang PF, Wu SHK, Shi XY, Deng BM, Sun C (1998) The aggregation behavior of chitosan bioelectret in aqueous solution using a fluorescence probe. J Mater Sci 33:1753–1757
Xie WM, Xu PX, Wang W, Liu Q (2002) Preparation and antibacterial activity of a water-soluble chitosan derivative. Carbohyd Polym 50:35–40
Chen L, Du Y, Tian Z, Sun L (2005) Effect of the degree of deacetylation and the substitution of carboxymethyl chitosan on its aggregation behavior. J Polym Sci Polym Phys 43:296–305
Zhang H, Zhou Z, Yang B, Gao M (2003) The influence of carboxyl groups on the photoluminescence of mercaptocarboxylic acid-stabilized CdTe nanoparticles. J Phys Chem B 107:8–13
He Y, Sai LM, Lu HT, Hu M, Lai WY, Fan QL, Wang LH, Huang W (2007) Microwave-assisted synthesis of water-dispersed CdTe nanocrystals with high luminescent efficiency and narrow size distribution. J Chem Mater 19:359–365
He Y, Lu H, Sai L, Lai W, Fan Q, Wang L, Huang W (2006) Synthesis of CdTe nanocrystals through program process of microwave irradiation. J Phys Chem B 110:13352–13356
Wuister SF, Driel F, Meijerink A (2003) Luminescence and growth of CdTe quantum dots and clusters. Phys Chem Chem Phys 5:1253–1258
Bao H, Gong Y, Li Z, Gao M (2004) Enhancement effect of illumination on the photoluminescence of water-soluble CdTe nanocrystals: toward highly fluorescent CdTe/CdS core−shell structure. Chem Mater 16:3853–3859
Wang Y, Tang Z, Correa-Duarte MA, Pastoriza-Santos I, Giersig M, Kotov NA, Liz-Marzan LM (2004) Mechanism of strong luminescence photoactivation of citrate-stabilized water-soluble nanoparticles with CdSe cores. J Phys Chem B 108:15461–15496
Lin YW, Hsieh MM, Liu CP, Chang HT (2005) Photoassisted synthesis of CdSe and core−shell CdSe/CdS quantum dots. Langmuir 21:728–734
Aldana J, Wang A, Peng XJ (2001) Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. Am Chem Soc 123:8844–8850
Talapin DV, Rogach AL, Shevchenko EV, Kornowski A, Haase M, Weller H (2002) Dynamic distribution of growth rates within the ensembles of colloidal II–VI and III–V semiconductor nanocrystals as a factor governing their photoluminescence efficiency. J Am Chem Soc 124:5782–5790
Acknowledgment
This work was supported by National High Technology Research and Development Program 863 of China (No. 2007AA06Z418).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, Z., Zhu, H. & Zhou, P. Microwave-Assisted Aqueous Synthesis of Highly Luminescent Carboxymethyl Chitosan-Coated CdTe/CdS Quantum Dots as Fluorescent Probe for Live Cell Imaging. J Fluoresc 22, 193–199 (2012). https://doi.org/10.1007/s10895-011-0946-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-011-0946-8