Abstract
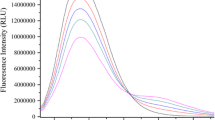
The interactions between potassium mono and di phosphates and bovine serum albumin (BSA) were studied using fluorescence spectroscopy (FS) and ultraviolet spectroscopy (UV). The experimental results showed that the potassium mono and di phosphates could insert into the BSA and quench the inner fluorescence of BSA by forming the potassium mono phosphate—BSA and pottassium di phosphate—BSA complexes. It was found that the static quenching was the main reason leading to the fluorescence quenching. It was conformed by XRD and SEM techniques.











Similar content being viewed by others
References
Boghaci DM, Farvid SS, Gharagozlou M (2007) Interaction of copper (11) complex of compartmental schiff base ligand N, N’—bis (3-hydroxysalicylindene ethylenediamine with bovine serum albumin. Spectrochim Acta A 66:650–655
Foster JF (1960) The Plasma Proteins (F.W. Putnam. Ed.) Vol. 1. Academic Press, New York/London, pp 179–239
Spector AA (1975) Fatty acid binding to plasma albumin. J Lipid Res 16:165–179
Reed RG, Feldhoff RC, Clute OL, Peters T (1975) Fragments of Bovine serum albumin produced by limited proteolysis, conformation and Ligand Binding. Biochemistry 14:4578–4583
Brand L, Gohlke JR (1972) Fluorescence probes for structure. Annu Rev Biochem 41:843–868
Silva D, Cortez CM, Chunha-Bastos J, Loura Sonia RW (2004) Methyl parathion interaction with human and bovine serum albumin. Toxicol Lett 147:53–61
Athina P, Green Rebecca J, Frazier Richard A (2005) Interaction of flavonoids with bovine serum albumin: a fluorescence quenching study. J Agric Food Chem 53:158–63
Lakowicz JR (1999) Principles of fluorescence spectroscopy, 2nd edn. Kluwer Academic Publishers/Plenum Press, New York
Romer J, Bichkel MH (1979) Mehtod to estimate binding constants at variable protein concentrations. J Pharm Pharmacol 31:7–11
Bhattacharyya M, Chauahuri V, Poddar RK (1990) Evidence for co operative Binding of chloropromazine with hemoglobin: equilibrium dialysis, fluorescence quenching and oxygen release study. Biochem Biophys Res Commun 167:1146–1153
Zhao H, Su W, Luo YH, Li ZC, Jiu HF, Liang H, Chen B, Zhang QJ (2006) Rectification of excitation with bathochromic shift induced by intense absorption of organic ligands during emission. Spectrochim Acta A 65:846–851
Subbiah D, Ashok KM (2005) Fluorescence spectroscopic study of serum albumin-bromadiolone interaction: fluorimetric determination of bromadiolone. J Pharm Biomed Anal 38:556–563
Hirshfactd KM, Toptygin D, Grandhige G, Kim H, Packard BZ, Brand C (1996) Steady-state and time—resolved fluorescence measurements for studying molecular interactions: interaction of a calcium—binding probe with proteins. Bio Phys Chem 62:25–38
Richeieri GV, Ancl A, Kleinfeld AM (1993) Interaction of long-chain fatty acids and albumin: determination of free fatty acid levels using the fluorescent probe ADIFAR. Biochemistry 32:7574–7580
Olson MK, Hollingworth S, Baylor SM (1988) Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J 54:1089–1104
Kuresbayashi N, Harkins AB, Baylor SM (1993) Use of fura red as an intracellular calcium indicator in frog skeletal muscle fibres. Biophys J 64:1934–1960
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakkialakshmi, S., Shanthi, B. & Chandrakala, D. Interaction of Potassium Mono and Di Phosphates with Bovine Serum Albumin Studied by Fluorescence Quenching Method. J Fluoresc 21, 687–692 (2011). https://doi.org/10.1007/s10895-010-0756-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-010-0756-4