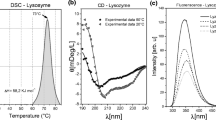
Abstract
The study of protein conformational changes in the presence of surfactants and lipids is important in the context of protein folding and misfolding. In the present study, we have investigated the mechanism of the protein conformational change coupled with aggregation leading to size growth of Hen Egg White Lysozyme (HEWL) in the presence of an anionic detergent such as sodium dodecyl sulphate (SDS) in alkaline pH. We have utilized intrinsic protein fluorescence (tryptophan) and extrinsic fluorescent reporters such as 8-anilinonaphthalene-1-sulfonic acid (ANS), dansyl and fluorescein to follow the protein conformational change in real-time. By analyzing the kinetics of fluorescence intensity and anisotropy of multiple fluorescent reporters, we have been able to delineate the mechanism of surfactant-induced aggregation of lysozyme. The kinetic parameters reveal that aggregation proceeds with an initial fast-phase (conformational change) followed by a slow-phase (self-assembly). Our results indicate that SDS, below critical micelle concentration, induces conformational expansion that triggers the aggregation process at a micromolar protein concentration range.







Similar content being viewed by others
References
Luheshi LM, Crowther DC, Dobson CM (2008) Protein misfolding and disease: from the test tube to the organism. Curr Opin Chem Biol 12:25–31
Soto C (2003) Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 4:49–60
Pepys MB, Hawkins PN, Booth DR, Vigushin DM, Tennent GA, Soutar AK, Totty N, Nguyen O, Blake CCF, Terry CJ, Feest TG, Zalin AM, Hsuan JJ (1993) Human Lysozyme gene mutation cause hereditary amyloidosis. Nature 362:553–557
Uversky VN, Fink AL (2004) Conformational constraints for amyloid fibrillation: the importance of being unfolded. Biochim Biophys Acta 1698:131–153
Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300:486–489
Kumar S, Mohanty SK, Udgaonkar JB (2007) Mechanism of formation of amyloid protofibrils of barstar from soluble oligomers: evidence for multiple steps and lateral association coupled to conformational conversion. J Mol Biol 367:1186–1204
Sluzky V, Tamada JA, Klibanov AM, Langer R (1991) Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc Natl Acad Sci USA 88:9377–9381
Ferrão-Gonzales AD, Souto SO, Silva JL, Foguel D (2000) The preaggregated state of an amyloidogenic protein: hydrostatic pressure converts native transthyretin into the amyloidogenic state. Proc Natl Acad Sci USA 97:6445–6450
Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M (2002) Inherent toxicity of aggregates implies a common mechanism for misfolded disease. Nature 416:507–511
Nilsson MR (2004) Techniques to study amyloid fibril formation in vitro. Methods 34:151–160
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Kluwer Academic/Plenum, New York
Fluorescence Spectroscopy in Biology (2005) Hof M, Hutterer R, Fidler V (Eds.) Vol. 3, Springer, Berlin Heidelberg
Nilsson MR, Dobson CM (2003) In vitro characterization of lactoferrin aggregation and amyloid formation. Biochemistry 42:375–382
Sasahara K, Yagi H, Sakai M, Naiki M, Goto Y (2008) Amyloid nucleation triggered by agitation of β2-microglobulin under acidic and neutral pH conditions. Biochemistry 47:2650–2660
Jiménez JL, Nettleton EJ, Bouchard M, Robinson CV, Dobson CM, Saibil HR (2002) The protofilament structure of insulin amyloid fibrils. Proc Natl Acad Sci USA 99:9196–9201
Padrick SB, Miranker AD (2002) Islet amyloid: phase partitioning and secondary nucleation are central to the mechanism of fibrillogenesis. Biochemistry 41:4694–4703
Mukhopadhyay S, Nayak P, Udgaonkar JB, Krishnamoorthy G (2006) Characterization of amyloid fibril formation by mapping residue-specific fluorescence dynamics. J Mol Biol 358:935–942
Itzhaki LS, Evans PA, Dobson CM, Radford SE (1994) Tertiary interactions in the folding pathway of hen lysozyme: kinetic studies using fluorescent probes. Biochemistry 33:5212–5220
Rothwarf DM, Scheraga HA (1996) Role of non-native aromatic and hydrophobic interactions in the folding of hen egg white lysozyme. Biochemistry 35:13797–13807
Bachmann A, Segel D, Kiefhaber T (2002) Test for cooperativity in the early kinetic intermediate in lysozyme folding. Biophys Chem 96:141–151
Booth DR, Sunde M, Bellotti V, Robinson CV, Hutchinson WL, Fraser PE, Hawkins PN, Dobson CM, Radford SE, Blake CCF, Pepys MB (1997) Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature 385:787–793
Dumoulin M, Kumita JR, Dobson CM (2006) Normal and aberrant biological self-assembly: insights from studies of human lysozyme and its amyloidogenic variants. Acc Chem Res 39:603–610
Merlini G, Bellotti V (2005) Lysozyme: a paradigmatic molecule for the investigation of protein structure, function and misfolding. Clin Chim Acta 357:168–172
Frare E, Mossuto MF, de Laureto PP, Tolin S, Menzer L, Dumoulin M, Dobson CM, Fontana A (2009) Characterization of oligomeric species on the aggregation pathway of human lysozyme. J Mol Biol 387:17–27
Canet D, Sunde M, Last AM, Miranker A, Spencer A, Robinson CV, Dobson CM (1999) Mechanistic studies of the folding of human lysozyme and the origin of amyloidogenic behavior in its disease-related variants. Biochemistry 38:6419–6427
Frare E, de Laureto PP, Zurdo J, Dobson CM, Fontana A (2004) A highly amyloidogenic region of hen lysozyme. J Mol Biol 340:1153–1165
Morshedi D, Ebrahim-Habibi A, Moosavi-Movahedi AA, Nemat-Gorgani M (2010) Chemical modification of lysine residues in lysozyme may dramatically influence its amyloid fibrillation. Biochim Biophys Acta 1804:714–722
Malisauskas M, Ostman J, Darinskas A, Zamotin V, Liutkevicius E, Lundgren E, Morozova-Roche LA (2005) Does the cytotoxic effect of transient amyloid oligomers from common equine lysozyme in vitro imply innate amyloid toxicity? J Biol Chem 280:6269–6275
Gharibyan AL, Zamotin V, Yanamandra K, Moskaleva OS, Margulis BA, Kostanyan IA, Morozova-Roche LA (2007) Lysozyme amyloid oligomers and fibrils induce cellular death via different apoptotic/necrotic pathways. J Mol Biol 365:1337–1349
Holley M, Eginton C, Schaefer D, Brown LR (2008) Characterization of amyloidogenesis of hen egg lysozyme in concentrated ethanol solution. Biochem Biophys Res Commun 373:164–168
Moosavi-Movahedi AA, Pirzadeh P, Hashemnia S, Ahmadian S, Hemmateenejad B, Amani M, Saboury AA, Ahmad F, Shamsipur M, Hakimelahi GH, Tsai FY, Alijanvand HH, Yousefi R (2007) Fibril formation of lysozyme upon interaction with sodium dodecyl sulfate at pH 9.2. Colloids Surf B Biointerfaces 60:55–61
Kumar S, Ravi VK, Swaminathan R (2008) How do surfactants and DTT affect the size, dynamics, activity and growth of soluble lysozyme aggregates? Biochem J 415:275–288
Vernaglia BA, Huang J, Clark ED (2004) Guanidine hydrochloride can induce amyloid fibril formation from hen egg-white lysozyme. Biomacromolecules 5:1362–1370
Pertinhez TA, Bouchard M, Smith RAG, Dobson CM, Smith LJ (2002) Stimulation and inhibition of fibril formation by a peptide in the presence of different concentrations of SDS. FEBS Lett 529:193–197
Andersen KK, Oliveira CL, Larsen KL, Poulsen FM, Callisen TH, Westh P, Pedersen JS, Otzen D (2009) The role of decorated SDS micelles in sub-CMC protein denaturation and association. J Mol Biol 391:207–226
Stenstam A, Khan A, Wennerström H (2001) The lysozyme-dodecyl sulfate system. An example of protein-surfactant aggregation. Langmuir 17:7513–7520
Chatterjee A, Moulik SP, Majhib PR, Sanyal SK (2002) Studies on surfactant—biopolymer interaction. I. Microcalorimetric investigation on the interaction of cetyltrimethylammonium bromide (CTAB) and sodium dodecylsulfate (SDS) with gelatin (Gn), lysozyme (Lz) and deoxyribonucleic acid (DNA). Biophys Chem 98:313–327
Pirzadeh P, Moosavi-Movahedi AA, Hemmateenejad B, Ahmad F, Shamsipur M, Saboury AA (2006) Chemometric studies of lysozyme upon interaction with sodium dodecyl sulfate and β-cyclodextrin. Colloids Surf B Biointerfaces 52:31–38
Yamashita S, Nishimoto E, Szabo AG, Yamasaki N (1996) Steady-state and time-resolved fluorescence studies on the ligand-induced conformational change in an active lysozyme derivative, Kyn62-Lysozyme. Biochemistry 35:531–537
Chowdhury RP, Chatterji D (2007) Estimation of Förster’s distance between two ends of Dps protein from mycobacteria: distance heterogeneity as a function of oligomerization and DNA binding. Biophys Chem 128:19–29
Semisotnov GV, Rodionova NA, Razgulyaev OI, Uversky VN, Gripas AF, Gilmanshin RI (1991) Study of the ‘molten globule’ intermediate state in protein folding by a hydrophobic fluorescent probe. Biopolymers 31:119–128
Lindgren M, Sörgjerd K, Hammarström P (2005) Detection and characterization of aggregates, prefibrillar amyloidogenic oligomers, and protofibrils using fluorescence spectroscopy. Biophys J 88:4200–4212
Fuguet E, Ráfols C, Rosés M, Bosch M (2005) Critical micelle concentration of surfactants in aqueous buffered and unbuffered systems. Anal Chim Acta 548:95–100
Wu LZ, Sheng YB, Xie JB, Wang W (2008) Photoexcitation of tryptophan groups induced reduction of disulfide bonds in hen egg white lysozyme. J Mol Struct 882:101–106
Frieden C (2007) Protein aggregation processes: in search of the mechanism. Protein Sci 16:2334–2344
Acknowledgements
We thank IISER Mohali for financial support during the course of the present investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, N., Bhattacharya, M. & Mukhopadhyay, S. Kinetics of Surfactant-induced Aggregation of Lysozyme Studied by Fluorescence Spectroscopy. J Fluoresc 21, 615–625 (2011). https://doi.org/10.1007/s10895-010-0749-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-010-0749-3