Abstract
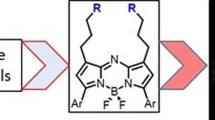
Derivatives of 4,4-difluoro-4-bora-3a,4a,diaza-s-indacene (BODIPY® or BDP) that possess a hydrazine substituent on position 5 are potential “turn-on” fluorophores for labeling aldehydes The unnatural amino acid L-3-formyltyrosine can be incorporated into a protein or peptide; thus, these hydrazines are potentially site specific labels for such polymers. In this work, model compounds were synthesized to assess whether the photochemical properties of the BDP-hydrazone would be suitable for protein labeling. Hydrazones were synthesized from the fluorophore 3-chloro-5-hydrazino-BDP and different aldehydes, and the absorption and emission spectra of the products were compared. The hydrazone of an unsubstituted aromatic aldehyde displays absorption and emission maxima (531 nm and 559 nm, respectively in dioxane) that are red shifted relative to those of a hydrazone from an aliphatic aldehyde (513 nm and 543 nm, respectively, in dioxane) and an increased quantum yield (0.21 vs. 0.11, respectively, in dioxane). The presence of a hydroxyl group ortho- to the aldehyde produces a hydrazone in which the absorption and emission maxima are slightly red shifted (528 nm and 564 nm, respectively in dioxane) from the unsubstituted aromatic hydrazone, but the quantum yields of the two hydrazones are equivalent. Thus, an ortho-hydroxy substituted aromatic aldehyde is a suitable electrophile for “turn on” protein labeling using the hydrazino-BDP. The specificity of this labeling reaction for the unnatural amino acid was demonstrated through fluorescent labeling of just the 3-formyltyrosine-containing α-subunit of α,β-tubulin.







Similar content being viewed by others
References
Loudet A, Burgess K (2007) BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem Rev 107:4891–4932
Ziessel R, Ulrich G, Harriman A (2007) The chemistry of Bodipy: A new El Dorado for fluorescence tools. New J Chem 31:496–501
Rohand T, Baruah M, Qin WW, Boens N, Dehaen W (2006) Functionalisation of fluorescent BODIPY dyes by nucleophilic substitution. Chem Commun 266–268.
Qin WW, Rohand T, Baruah M, Stefan A, Van der Auweraer M, Dehaen W, Boens N (2006) Solvent-dependent photophysical properties of borondipyrromethene dyes in solution. Chem Phys Lett 420:562–568
van Swieten PF, Leeuwenburgh MA, Kessler BM, Overkleeft HS (2005) Bioorthogonal organic chemistry in living cells: novel strategies for labeling biomolecules. Org Biomol Chem 3:20–27
Prescher JA, Bertozzi CR (2005) Chemistry in living systems. Nature Chem Biol 1:13–21
Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR (2006) A comparative study of bioorthogonal reactions with azides. ACS Chem Biol 1:644–648
Wang CCY, Seo TS, Li ZM, Ruparel H, Ju JY (2003) Site-specific fluorescent labeling of DNA using Staudinger ligation. Bioconjug Chem 14:697–701
Smith PAS (1983) Derivatives of Hydrazine and Other Hydronitrogens Having N-N Bonds. The Benjamin/Cummings Publishing Company Inc, Canada
Dilek Ö, Bane SL (2008) Synthesis of boron dipyrromethene fluorescent probes for bioorthogonal labeling. Tetrahedron Lett 49:1413–1416
Banerjee A, Panosian TD, Mukherjee K, Ravindra R, Gal S, Sackett DL, Bane, SL (2010) Site-specific orthogonal labeling of carboxy terminus of alpha –tubulin. ACS Chem Biol 5 (in press).
Ball P, Nicholls CH (1982) Azo-hydrazone tautomerism of hydroxyazo compounds—a review. Dyes Pigm 3:5–26
Shanker N, Bane SL (2008) Basic aspects of absorption and fluorescence spectroscopy and resonance energy transfer methods. Methods Cell Biol 84:213–242
Williams RC, Lee JC (1982) Preparation of tubulin from brain. Methods in Enzymol 85 Pt B:376–85.
Penefsky HS (1977) Reversible binding of inorganic phosphate by beef heart mitochondrial adenosine triphosphatase. J Biol Chem 252:2891–2899
Qin WW, Leen V, Dehaen W, Cui J, Xu C, Tang XL, Liu WS, Rohand T, Beljonne D, Van Averbeke B, Clifford JN, Driesen K, Binnemans K, Van der Auweraer M, Boens N (2009) 3, 5-Dianilino substituted difluoroboron dipyrromethene: Synthesis, spectroscopy, photophysics, crystal structure, electrochemistry, and quantum-chemical calculations. J Phys Chem C 113:11731–11740
Liras M, Prieto JB, Pintado-Sierra M, Arbeloa FL, Garcia-Moreno I, Costela AS, Infantes L, Sastre R, Amat-Guerri F (2007) Synthesis, photophysical properties, and laser behavior of 3-amino and 3-acetamido BODIPY dyes. Org Lett 9:4183–4186
Qin W, Baruah M, Van der Auweraer M, De Schryver FC, Boens N (2005) Photophysical properties of borondipyrromethene analogues in solution. J Phys Chem A 109:7371–7384
Jencks WP (1964) Mechanism and catalysis of simple carbonyl group reactions. Prog Phys Org Chem 2:63–128
Harej M, Dolenc D (2007) Autoxidation of hydrazones. Some new insights. J Org Chem 72:7214–7221
Acknowledgments
Thanks to Dr. Jürgen Schulte for helping collecting 11B NMR spectra and to Dr. Abhijit Banerjee for providing the formyltyrosinated tubulin and for assistance with the protein. We thank Professor Rebecca Kissling for helpful discussions and valuable scientific assistance. We also thank to David Tuttle for photography. Financial support from NIH (R01 CA69571 and R15 GM 093941) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dilek, O., Bane, S.L. Synthesis and Spectroscopic Characterization of Fluorescent Boron Dipyrromethene-Derived Hydrazones. J Fluoresc 21, 347–354 (2011). https://doi.org/10.1007/s10895-010-0723-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-010-0723-0