Abstract
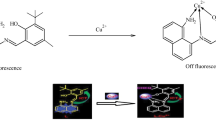
Two highly photostable yellow–green emitting 1,8-naphthalimides 5 and 6, containing both N-linked hindered amine moiety and a secondary or tertiary cation receptor, were synthesized for the first time. Novel compounds were configured as “fluorophore–spacer–receptor” systems based on photoinduced electron transfer. Photophysical characteristics of the dyes were investigated in DMF and water/DMF (4:1, v/v) solution. The ability of the new compounds to detect cations was evaluated by the changes in their fluorescence intensity in the presence of metal ions (Cu2+, Pb2+, Zn2+, Ni2+, Co2+) and protons. The presence of metal ions and protons was found to disallow a photoinduced electron transfer leading to an enhancement in the dye fluorescence intensity. Compound 5, containing secondary amine receptor, displayed a good sensor activity towards metal ions and protons. However the sensor activity of dye 6, containing a tertiary amine receptor and a shorter hydrocarbon spacer, was substantially higher. The results obtained indicate the potential of the novel compounds as highly photostable and efficient “off–on” pH switchers and fluorescent detectors for metal ions with pronounced selectivity towards Cu2+ ions.
















Similar content being viewed by others
Abbreviations
- HALS:
-
hindered amine light stabilizer
- PET:
-
photoinduced electron transfer
References
Balzani V (2003) Photochemical molecular devices. Photochem Photobiol Sci 2:459–476 doi:10.1039/b300075n
de Silva A, Fox D, Huxley A, Moody T (2000) Combining luminescence, coordination and electron transfer for signalling purposes. Coord Chem Rev 205:41–57 doi:10.1016/S0010-8545(00)00238-1
Rurack K, Resch-Gender U (2002) Rigidization, preorientation and electronic decoupling—the magic triangle for the design of highly efficient fluorescent sensors and switches. Chem Soc Rev 31:116–127 doi:10.1039/b100604p
Balzani V, Credi A, Raymo F, Stoddart J (2000) Artificial molecular machines. Angew Chem Int Ed 39:3348–3391 doi:10.1002/1521-3773(20001002)39:19<3348::AID-ANIE3348>3.0.CO;2-X
Raymo F (2002) Digital processing and communication with molecular switches. Adv Mater 14:401–414 doi:10.1002/1521-4095(20020318)14:6<401::AID-ADMA401>3.0.CO;2-F
He H, Mortellaro M, Leiner M, Young S, Fraatz R, Tusa J (2003) A fluorescent chemosensor for sodium based on photoinduced electron transfer. Anal Chem 75:549–555 doi:10.1021/ac0205107
Gunnlaugsson T, Bichell B, Nolan C (2002) A novel fluorescent photoinduced electron transfer (PET) sensor for lithium. Tetrahedron Lett 43:4989–4992 doi:10.1016/S0040-4039(02)00895-X
Bissell R, de Silva A, Gunaratne H, Lynch P, Maguire G, McCoy C et al (1992) Molecular fluorescent signalling with fluorophore–spacer–receptor systems: approaches to sensing and switching devices via supramolecular photophysics. Chem Soc Rev 21:187–196 doi:10.1039/cs9922100187
de Silva A, Gunaratne H, Gunnlaugsson T, Huxley A, McCoy C, Rademacher J et al (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566 doi:10.1021/cr960386p
Valeur B, Leray I (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205:3–40 doi:10.1016/S0010-8545(00)00246-0
de Silva A, Fox D, Huxley A, McClenaghan N, Roiron J (1999) Metal complexes as components of luminescent signalling systems. Coord Chem Rev 186:297–306 doi:10.1016/S0010-8545(98)00275-6
de Silva A, McCaughan B, McKinney B, Querol M (2003) Newer optical based molecular devices from older coordination chemistry. Dalton Transactions 10:1902–1913 doi:10.1039/b212447p
Callan J, de Silva A, Magri D (2005) Luminescent sensors and switches in the early 21st century. Tetrahedron 61:8551–8588 doi:10.1016/j.tet.2005.05.043
Gan J, Chen K, Chang CP, Tian H (2003) Luminescent properties and photo-induced electron transfer of naphthalimides with piperazine substituent. Dyes Pigm 57:21–28 doi:10.1016/S0143-7208(02)00162-6
de Silva A, Goligher A, Gunaratne H, Rice T (2003) The pH-dependent fluorescence of pyridylmethyl-4-amino-1,8-naphthalimides. Arkivoc 7:229–243
Patrick L, Whiting A (2002) Synthesis of some polymerisable fluorescent dyes. Dyes Pigm 55:123–132 doi:10.1016/S0143-7208(02)00067-0
Bojinov V, Konstantinova T (2002) Synthesis of polymerizable 1,8-naphthalimide dyes containing hindered amine fragment. Dyes Pigm 54:239–245 doi:10.1016/S0143-7208(02)00047-5
Bojinov V, Grabchev I (2003) Synthesis of new polymerizable 1,8-naphthalimide dyes containing a 2-hydroxyphenylbenzotriazole fragment. Dyes Pigm 59:277–283 doi:10.1016/S0143-7208(03)00113-X
Bojinov V, Panova I (2007) Synthesis and absorption properties of new yellow-green emitting benzo[de]isoquinoline-1,3-diones containing hindered amine and 2-hydroxyphenylbenzotriazole fragments. Dyes Pigm 74:551–560 doi:10.1016/j.dyepig.2006.03.016
Hrdlovič P, Chmela Š, Danko M (1998) Spectral characteristics and photochemical stability of fluorescence probes based on 1,8-naphthaleneimide in solution and in polymer matrix. J Photochem Photobiol A Chem 112:197–203 doi:10.1016/S1010-6030(97)00277-3
Kollar J, Hrdlovič P, Chmela Š (2008) Synthesis and spectral characteristics of di-substituted 1,8-naphthalimides: bi-radical formation. J Photochem Photobiol A Chem 195:64–71 doi:10.1016/j.jphotochem.2007.09.008
Hrdlovič P, Chmela Š, Danko M, Sarakha M, Guyot G (2008) Spectral properties of probes containing benzothioxanthene chromophore linked with hindered amine in solution and in polymer matrices. J Fluoresc 18:393–402 doi:10.1007/s10895-007-0279-9
Martin E, Weigand R, Pardo A (1996) Solvent dependence of the inhibition of intramolecular charge-transfer in N-substituted 1,8-naphthalimide derivatives as dye lasers. J Lumin 68:157–164 doi:10.1016/0022-2313(96)00008-7
Gruzinskii V, Kukhto A, Shakkah G (1998) Spectra of lasing efficiency in lasers with solutions of complex organic compounds. J Appl Spectrosc 65:463–465 doi:10.1007/BF02675471
Tao ZF, Qian X (1999) Naphthalimide hydroperoxides as photonucleases: substituent effects and structural basis. Dyes Pigm 43:139–145 doi:10.1016/S0143-7208(99)00037-6
Stewart W (1981) Synthesis of 3,6-disulfonated 4-aminonaphthalimides. J Am Chem Soc 103:7615–7620 doi:10.1021/ja00415a033
Morgado J, Gruner J, Walcott SP, Yong TM, Cervini R, Moratti SC et al (1998) 4-AcNI—a new polymer for light-emitting diodes. Synth Met 95:113–117 doi:10.1016/S0379-6779(98)00042-3
Zhu W, Hu C, Chen K, Tian H (1998) Luminescent properties of copolymeric dyad compounds containing 1,8-naphthalimide and 1,3,4-oxadiazole. Synth Met 96:151–154 doi:10.1016/S0379-6779(98)00083-6
Tian H, Gan J, Chen K, He J, Song Q, Hou X (2002) Positive and negative fluorescent imaging induced by naphthalimide polymers. J Mater Chem 12:1262–1267 doi:10.1039/b200509c
Grabchev I, Qian X, Bojinov V, Xiao Y, Zhang W (2002) Synthesis and photophysical properties of 1,8-naphthalimide-labelled dendrimers as PET sensors of proton and transition metal ion. Polymer (Guildf) 43:5731–5736 doi:10.1016/S0032-3861(02)00417-2
Tian H, Xu T, Zhao Y, Chen K (1999) Two-path photo-induced electron transfer in naphthalimide-based model compound. J Chem Soc Perkin Trans 2:545–549 doi:10.1039/a808123i
Bojinov V, Konstantinova T (2007) Fluorescent 4-(2,2,6,6-tetramethylpiperidin-4-ylamino)-1,8-naphthalimide pH chemosensor based on photoinduced electron transfer. Sens Actuators B Chem 123:869–876 doi:10.1016/j.snb.2006.10.035
Poteau X, Brown A, Brown R, Holmes C, Matthew D (2000) Fluorescence switching in 4-amino-1,8-naphthalimides: “on–off–on” operation controlled by solvent and cations. Dyes Pigm 47:91–105 doi:10.1016/S0143-7208(00)00067-X
Jia L, Zhang Y, Guo X, Qian X (2004) A novel chromatism switcher with double receptors selectively for Ag+ in neutral aqueous solution: 4,5-diaminoalkeneamino-N-alkyl-l,8-naphthalimides. Tetrahedron Lett 45:3969–3973 doi:10.1016/j.tetlet.2004.03.105
Zhu W, Hu M, Yao R, Tian H (2003) A novel family of twisted molecular luminescent materials containing carbazole unit for single-layer organic electroluminescent devices. J Photochem Photobiol A Chem 154:169–177 doi:10.1016/S1010-6030(02)00325-8
Facoetti H, Robin P, Le Barny P, Schott M, Bouche CM, Berdague P (1996) Side-chain electroluminescent polymers. Synth Met 81:191–195 doi:10.1016/S0379-6779(96)03767-8
Zhu W, Minami N, Kazaoui S, Kim Y (2003) Fluorescent chromophores functionalized single-wall carbon nanotubes with minimal alteration to their characteristic one-dimensional electronic states. J Mater Chem 13:2196–2201 doi:10.1039/b303885h
Grabchev I, Chovelon JM (2003) Synthesis and functional properties of green fluorescent poly(methylmethacrylate) for use in liquid crystal systems. Polym Adv Technol 14:601–608 doi:10.1002/pat.376
Grabchev I, Moneva I, Bojinov V, Guittonneau S (2000) Synthesis and properties of fluorescent 1,8-naphthalimide dyes for application in liquid crystal displays. J Mater Chem 10:1291–1296 doi:10.1039/a909153j
Cosnard F, Wintgens V (1998) A new fluoroionophore derived from 4-amino-N-methyl-1,8-naphthalimide. Tetrahedron Lett 39:2751–2754 doi:10.1016/S0040-4039(98)00302-5
Grabchev I, Sali S, Betcheva R, Gregoriou V (2007) New green fluorescent polymer sensors for metal cations and protons. Eur Polym J 43:4297–4305 doi:10.1016/j.eurpolymj.2007.07.036
Reynolds G, Drexhage K (1975) New coumarin dyes with rigidized structure for flashlamp-pumped dye lasers. Opt Commun 13:222–225 doi:10.1016/0030-4018(75)90085-1
de Silva A, Gunaratne H, McCoy C (1993) A molecular photoionic AND gate based on fluorescent signaling. Nature 364:42–44 doi:10.1038/364042a0
de Sivla A, Gunaratne H, Habib-Jiwan JL, McCoy C, Rice T, Soumillion JP (1995) New fluorescent model compounds for the study of photoinduced electron transfer: the influence of molecular electric field in the excited state. Angew Chem Int Ed Engl 34:1728–1731 doi:10.1002/anie.199517281
Rurack K (2001) Flipping the light switch ‘ON’—the design of sensor molecules that show cation-induced fluorescence enhancement with heavy and transition metal ions. Spectrochim Acta Part A Mol Biomol Spectrosc 57:2161–2195
Gordon P, Gregory P (1987) Organic chemistry in colour. Springer, Berlin
Grabchev I, Bojinov V, Petkov H (2001) Synthesis and photophysical properties of polymerizable 1,8-naphthalimide dyes and their copolymers with styrene. Dyes Pigm 51:1–8 doi:10.1016/S0143-7208(01)00041-9
Yang S, Meng F, Tian H, Chen K (2002) Photostability of novel copolymers functionalized with laser dyes based on modified rhodamine 6G and 1,8-naphthalimide. Eur Polym J 38:911–919 doi:10.1016/S0014-3057(01)00265-8
Terenin A (1967) Photonica of dyes and related organic compounds. Science, Leningrad (in Russian)
Gunnlaugsson T, McCoy C, Morrow R, Phelan C, Stomeo F (2003) Towards the development of controllable and reversible ‘on–off’ luminescence switching in soft-matter; synthesis and spectroscopic investigation of 1,8-naphthalimide-based PET (photoinduced electron transfer) chemosensors for pH in water-permeable hydrogels. Arkivoc 7:216–228
Acknowledgements
This work was supported by the National Science Foundation of Bulgaria (project VU-X-201/06). Vladimir Bojinov and Nikolai Georgiev also acknowledge the Science Foundation at the University of Chemical Technology and Metallurgy (Sofia, Bulgaria).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bojinov, V.B., Georgiev, N.I. & Bosch, P. Design and Synthesis of Highly Photostable Yellow–Green Emitting 1,8-Naphthalimides as Fluorescent Sensors for Metal Cations and Protons. J Fluoresc 19, 127–139 (2009). https://doi.org/10.1007/s10895-008-0394-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-008-0394-2