Abstract
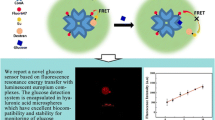
Poly(ethylene glycol) (PEG) hydrogels have been used to encapsulate fluorescently labeled molecules in order to detect a variety of analytes. The hydrogels are designed with a mesh size that will retain the sensing elements while allowing for efficient diffusion of small analytes. Some sensing assays, however, require a conformational change or binding of large macromolecules, which may be sterically prohibited in a dense polymer matrix. A process of hydrogel microporation has been developed to create cavities within PEG microspheres to contain the assay components in solution. This arrangement provides improved motility for large sensing elements, while limiting leaching and increasing sensor lifetime. Three hydrogel compositions, 100% PEG, 50% PEG, and microporated 100% PEG, were used to create pH-sensitive microspheres that were tested for response time and stability. In order to assess motility, a second, more complex sensor, namely a FITC-dextran/TRITC-Con A glucose-specific assay was encapsulated within the microspheres.





Similar content being viewed by others
Refrences
Yadavalli VK, Koh WG, Lazur GJ, Pishko MV (2004) Microfabricated protein-containing poly(ethylene glycol) hydrogel arrays for biosensing. Sensors Actuators B Chem 97(2–3):290–297
O’Neal DP, Meledeo MA, Davis JR, Ibey BL, Gant VA, Pishko MV, Coté GL (2004) Oxygen sensor based on the fluorescence quenching of a ruthenium complex immobilized in a biocompatible poly(ethylene glycol) hydrogel. IEEE Sensors J 4(6):728–734
Russell RJ, Pishko MV, Gefrides CC, McShane MJ, Coté GL (1999) A fluorescence-based glucose biosensor using concanavalin A and dextran encapsulated in a poly(ethylene glycol) hydrogel. Anal Chem 71(15):3126–3132
Kim SH, Kim B, Yadavalli VK, Pishko MV (2005) Encapsulation of enzymes within polymer spheres to create optical nanosensors for oxidative stress. Anal Chem 77(21):6828–6833
Russell RJ, Pishko MV, Simonian AL, Wild JR (2000) Poly(ethylene glycol) hydrogel-encapsulated fluorophore-enzyme conjugates for direct detection of organophosphorus neurotoxins. Abstracts of Papers of the American Chemical Society 219:U107–U107
Zguris J, Pishko MV (2005) pH sensitive fluorescent poly(ethylene) glycol hydrogel microstructures for monitoring in cell culture systems. Sens Lett 3(3):206–210
Heo J, Crooks RM (2005) Microfluidic biosensor based on an array of hydrogel-entrapped enzymes. Anal Chem 77(21):6843–6851
Sharma S, Johnson RW, Desai TA (2003) Ultrathin poly(ethylene glycol) films for silicon-based microdevices. Appl Surf Sci 206(1–4):218–229
Harris JM, Zalipsky S (1997) Poly(ethylene glycol): Chemistry and Biological Applications, in ACS Symposium Series, vol 680. American Chemical Society, Washington, DC, p 489
Rihova B (1996) Biocompatibility of biomaterials: Hemocompatibility, immunocompatibility and biocompatibility of solid polymeric materials and soluble targetable polymeric carriers. Adv Drug Deliv Rev 21(2):157–176
Quinn CAP, Connor RE, Heller A (1997) Biocompatible, glucose-permeable hydrogel for in situ coating of implantable biosensors. Biomaterials 18(24):1665–1670
Russell RJ, Axel AC, Shields KL, Pishko MV (2001) Mass transfer in rapidly photopolymerized poly(ethylene glycol) hydrogels used for chemical sensing. Polymer 42(11):4893–4901
Mellott MB, Searcy K, Pishko MV (2001). Release of protein from highly cross-linked hydrogels of poly(ethylene glycol) diacrylate fabricated by UV polymerization. Biomaterials 22(9):929–941
Kurdikar DL, Peppas NA (1995) The volume shrinkage, thermal and sorption behavior of polydiacrylates. Polymer 36(11):2249–2255
Ibey BL, Beier HT, Yadavalli VK, Rounds RM, Coté GL, Pishko MV (2005) Competitive binding assay for glucose based on glycodendrimer-fluorophore conjugates. Anal Chem 77(21):7039–7046
Schultz JS, Mansouri S, Goldstein IJ (1982) Affinity sensor – a new technique for developing implantable sensors for glucose and other metabolites. Diabetes Care 5(3):245–253
Meadows D, Schultz JS (1988) Fiber-optic biosensors based on fluorescence energy-transfer. Talanta 35(2):145–150
Meadows DL, Schultz JS (1993) Design, manufacture and characterization of an optical-fiber glucose affinity sensor-based on an homogeneous fluorescence energy-transfer assay system. Anal Chim Acta 280(1):21–30
Bittiger H, Schnebli HP (1976) Concanavalin a as a tool. John Wiley & Sons, London
Chowdhury TK, Weiss AK (1975) Concanavalin A, in Advances in experimental medicine and biology, vol 55, 2nd edn. Plenum Press, New York
Ibey BL, Meledeo MA, Gant VA, Yadavalli V, Pishko MV, Cote GL (2003) In vivo monitoring of blood glucose using poly(ethylene glycol) microspheres. Proc SPIE 49651-6
Ballerstadt R, Schultz JS (2000) A fluorescence affinity hollow fiber sensor for continuous transdermal glucose monitoring. Anal Chem 72(17):4185–4192
Ballerstadt R, Polak A, Beuhler A, Frye J (2004) In vitro long-term performance study of a near-infrared fluorescence affinity sensor for glucose monitoring. Biosens Bioelectron 19(8):905–914
Barone PW, Parker RS, Strano MS (2005) In vivo fluorescence detection of glucose using a single-walled carbon nanotube optical sensor: Design, fluorophore properties, advantages, and disadvantages. Anal Chem 77(23):7556–7562
Chinnayelka S, McShane MJ (2004) Glucose-sensitive nanoassemblies comprising affinity-binding complexes trapped in fuzzy microshells. J Fluorescence 14(5):585–595
Chinnayelka S, McShane MJ (2005) Microcapsule biosensors using competitive binding resonance energy transfer assays based on apoenzymes. Anal Chem 77(17):5501–5511
Chen J, Park H, Park K (1999) Synthesis of superporous hydrogels: Hydrogels with fast swelling and superabsorbent properties. J Biomed Mater Res 44(1):53–62
Oxley HR, Corkhill PH, Fitton JH, Tighe BJ (1993) Macroporous hydrogels for biomedical applications: methodology and morphology. Biomaterials 14(14):1064–1072
Badiger MV, McNeill ME, Graham NB (1993) Porogens in the preparation of microporous hydrogels based on poly(ethylene oxides). Biomaterials 14(14):1059–1063
Filmon R, Retailleau-Gaborit N, Grizon F, Galloyer M, Cincu C, Basle MF, Chappard D (2002) Non-connected versus interconnected macroporosity in poly(2-hydroxyethyl methacrylate) polymers. An X-ray microtomographic and histomorphometric study. J Biomater Sci Polym Ed 13(10):1105–1117
Yadavalli VK, Russell RJ, Pishko MV, McShane MJ, Coté GL (2005) A Monte Carlo simulation of photon propagation in fluorescent poly(ethylene glycol) hydrogel microsensors. Sensors Actuators B Chem 105(2):365–377
Canal T, Peppas NA (1989) Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J Biomed Mater Res 23:1183–1193
SAS Institute Inc. (2005) JMP Statistics and Graphics Guide. Cary, NC, pp 731–753
Shewhart W (1931) Economic control of quality of manufactured product. Van Nostrand, Princeton, NJ
Acknowledgments
The authors gratefully acknowledge support from the National Aeronautics and Space Administration (Grant NNJ04HB04G). H.T.B would like to acknowledge the support of a National Science Foundation Graduate Research Fellowship. B.L.I. acknowledges the support of a Graduate Assistance in Areas of National Need (GAANN) Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rounds, R.M., Ibey, B.L., Beier, H.T. et al. Microporated PEG Spheres for Fluorescent Analyte Detection. J Fluoresc 17, 57–63 (2007). https://doi.org/10.1007/s10895-006-0143-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-006-0143-3