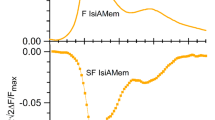
Flavoproteins are components of plasma membrane redox chains, which have been suggested to play major roles in neuronal activity and survival. We found that the red/orange autofluorescence of mature primary cultures of cerebellar granule neurons (8–9 days in vitro) was largely quenched by millimolar concentrations of dithionite added to the extracellular medium, and pointed out that nearly 50% of this autofluorescence was due to plasma membrane-bound flavoproteins. We report in this work that the lipophilic neuronal plasma membrane markers N-(3-triethylammoniumpropyl)-4-(4-(4-(diethylamino)phenyl)butadienyl)-pyridinium dibromide (RH-414) and N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino)phenyl)hexatrienyl)pyridinium dibromide (FM4-64) can form fluorescence energy transfer donor–acceptor pairs with flavoproteins with calculated R 0 values between 3.7 and 4.2 nm. The quantification of the efficiency of fluorescence energy transfer with different concentrations of acceptor dyes has been worked out with re-suspended neurons. Using quantitative images of the neurons in culture, acquired with a CCD camera attached to an epifluorescence microscope, regionalization of the plasma membrane-bound flavoproteins of cerebellar granule neurons has been achieved from the quenching by dithionite of the fluorescence of the acceptor dye. The results unraveled that plasma membrane-bound flavoproteins are largely enriched in interneuronal contact sites forming clusters of 0.5–1 μm diameter size, which appears largely regionalized in the neuron's cell body.






Similar content being viewed by others
REFERENCES
F. L. Crane, H. Löw, and M. G. Clark (1985). Transplasma-membrane redox system in growth and development. Biochim. Biophys. Acta 811(3), 233–266.
J. M. May (1999). Is ascorbic acid an antioxidant for the plasma membrane? FASEB J. 13(9), 995–1006.
Y. Yong and J. L. Dreyer (1995). Distribution of six transplasma membrane NADH-dehydrogenases in rat brain tissue. Brain Res. Dev. Brain Res. 89(2), 235–252.
J. L. Dreyer (1990). Plasma membrane dehydrogenases in rat brain synaptic membranes. Multiplicity and subunit composition. J. Bioenerg. Biomembr. 22(5), 619–633.
F. J. Martin-Romero, Y. Gutierrez-Martin, F. Henao, and C. Gutierrez-Merino (2002). The NADH oxidase activity of the plasma membrane of synaptosomes is a major source of superoxide anion and is inhibited by peroxynitrite. J. Neurochem. 82(3), 604–614.
F. J. Martin-Romero, E. Garcia-Martin, and C. Gutierrez-Merino (2002). Inhibition of oxidative stress produced by plasma membrane NADH oxidase delays low-potassium-induced apoptosis of cerebellar granule cells. J. Neurochem. 82(3), 705–715.
J. M. Villalba, F. Navarro, F. Cordoba, A. Serrano, A. Arroyo, F. L. Crane, and P. Navas (1995). Coenzyme Q reductase from liver plasma membrane: purification and role in trans-plasma-membrane electron transport. Proc. Natl. Acad. Sci. USA 92(11), 4887–4891.
C. Kim, F. L. Crane, W. P. Faulk, and D. J. Morre (2002). Purification and characterization of a doxorubicin-inhibited NADH-quinone (NADH-ferricyanide) reductase from rat liver plasma membranes. J. Biol. Chem. 277(19), 16441–16447.
A. Kindzelskii and H. R. Petty (2004). Fluorescence spectroscopic detection of mitochondrial flavoprotein redox oscillations and transient reduction of the NADPH oxidase-associated flavoprotein in leukocytes. Eur. Biophys. J. 33(4), 291–299.
J. E. Brenman and D. S. Bredt (1997). Synaptic signaling by nitric oxide. Curr. Opin. Neurobiol. 7(3), 374–378.
L. Eide, and C. T. McMurray (2005). Culture of adult mouse neurons. BioTechniques 38(1), 99–104.
C. Gutiérrez-Merino, F. Munkonge, A. M. Mata, J. M. East, B. L. Levinson, R. M. Napier, and A. G. Lee (1987). The position of the ATP binding site on the (Ca2+, + Mg2+)-ATPase. Biochim. Biophys. Acta 897(2), 207–216.
F. Centeno, and C. Gutiérrez-Merino (1992). Location of functional centers in the microsomal cytochrome P450 system. Biochemistry 31(36), 8473–8481.
C. Gutiérrez-Merino, I. C. Bonini de Romanelli, L. I. Pietrasanta, and F. J. Barrantes (1995). Preferential distribution of the fluorescent phospholipid probes NBD-phosphatidylcholine and rhodamine-phosphatidylethanolamine in the exofacial leaflet of acetylcholine receptor-rich membranes from Torpedo marmorata. Biochemistry 34(14), 4846–4855.
C. Gutiérrez-Merino (1981). Quantitation of the Forster energy transfer for two-dimensional systems. I. Lateral phase separation in unilamellar vesicles formed by binary phospholipid mixtures. Biophys. Chem. 14(3), 247–257.
C. Gutiérrez-Merino (1981). Quantitation of the Forster energy transfer for two-dimensional systems. II. Protein distribution and aggregation state in biological membranes. Biophys. Chem. 14(3), 259–266.
C. Gutiérrez-Merino, F. Centeno, E. Garcia-Martin, and J. M. Merino (1994). Fluorescence energy transfer as a tool to locate functional sites in membrane proteins. Biochem. Soc. Trans. 22(3), 784–788.
R. P. Haugland (2005). The Handbook: A Guide to Fluorescent Probes and Labeling Techniques, 10th ed., (editor Michelle T.Z. Spence), pages 585,587,676,788-989 and 976-978. Molecular Probes/Invitrogen, Carlsbad(California, USA).
D. Schild, H. Geiling, and J. Bischofberger (1995). Imaging of L-type Ca2+ channels in olfactory bulb neurones using fluorescent dihydropyridine and a styryl dye. J. Neurosci. Methods 59(2), 183–190.
R. A. McKinney (2005). Physiological roles of spine motility: development plasticity and disorders. Biochem. Soc. Trans. 33(6), 1299–1302.
A. K. Samhan-Arias, F. J. Martin-Romero, and C. Gutierrez-Merino (2004). Kaempferol blocks oxidative stress in cerebellar granule cells and reveals a key role for reactive oxygen species production at the plasma membrane in the commitment to apoptosis. Free Radic. Biol. Med. 37(1), 48–61.
R. Letters (1964). The application of a two-dimensional paper-chromatographic technique to the analysis of phospholipids. Biochem. J. 93, 313–316.
J. R. Lakowicz (1999). Principles of Fluorescence Spectroscopy 2nd ed, Kluwer Academic/Plenum Press, New York.
C. Gutiérrez-Merino, A. Molina, B. Escudero, A. Diez, and J. Laynez (1989). Interaction of the local anesthetics dibucaine and tetracaine with sarcoplasmic reticulum membranes. Differential scanning calorimetry and fluorescence studies. Biochemistry 28(8), 3398–3406.
P. I. Bastiaens, P. J. Bonants, F. Muller, and A. J. Visser (1989). Time-resolved fluorescence spectroscopy of NADPH-cytochromeP-450 reductase: demonstration of energy transfer between the two prosthetic groups. Biochemistry 28(21), 8416–8425.
J. Koziol (1971). Fluorometric analyses of riboflavin and its coenzymes. Methods Enzymol XVIII (Part B), 253–285.
W. H. Lawson, Jr., R. A. Holland, and R. E. Forster (1965). Effect of temperature on deoxygenation rate of human red cells. J. Appl. Physiol. 20(5), 912–918.
S. S. Antollini, M. A. Soto, I. Bonini de Romanelli, C. Gutiérrez-Merino, P. Sotomayor, and F. J. Barrantes (1996). Physical state of bulk and protein-associated lipid in nicotinic acetylcholine receptor-rich membrane studied by laurdan generalized polarization and fluorescence energy transfer. Biophys. J. 70(3), 1275–1284.
ACKNOWLEDGMENTS
This work has been supported by Grant SAF2003-08275 of the Spanish Ministerio de Ciencia y Tecnología. AKSA and MAGB are recipient of predoctoral fellowships of the Junta de Extremadura and Spanish Ministerio de Ciencia y Tecnología, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samhan-Arias, A.K., García-Bereguiaín, M.A., Martín-Romero, F.J. et al. Regionalization of Plasma Membrane-Bound Flavoproteins of Cerebellar Granule Neurons in Culture by Fluorescence Energy Transfer Imaging. J Fluoresc 16, 393–401 (2006). https://doi.org/10.1007/s10895-005-0065-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-005-0065-5