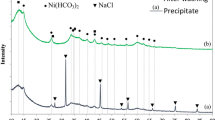
Zinc nanoparticles applied in the production of zinc–air batteries provide the highest specific energy among various metal–air combinations used in batteries in practice. Recent technical advances provide an opportunity to successfully solve the problems experienced during the early development of batteries of this type. For example, there is a major limitation in recharging cycles due to the massive formation of zinc oxide. In the present work, a reduction process is proposed on the basis of the use of alcohol solvent by applying microwave in-liquid plasma. The occurrence of reduction was confirmed by calculating the chemical potential of ethanol and methanol as well as spectra of plasma emission in the temperature range 1500–2000 K. Characterization of synthesized nanoparticles was carried out by energy-dispersive X-ray spectrometry and transmission electron microscopy.
Similar content being viewed by others
References
J.-S. Lee, S. Tai Kim, R. Cao R, N.-S. Choi, M. Liu, K. Tae Lee, and J. Cho, Metal–air batteries with high energy density: Li–air versus Zn–air, Adv. Energy Mater., 1, No. 1, 34–50 (2011).
V. Caramia and B. Bozzini, Materials science aspects of zinc–air batteries: A review, Mater. Renew. Sustain. Energy, 3, No. 2, 28–40 (2014).
Z. Hu, G. Oskam, and P. C. Searson, Influence of solvent on the growth of ZnO nanoparticles, J. Colloid Interface Sci., 263, No. 2, 454–460 (2003).
L. Obreja, N. Foca, M. I. Popa, and V. Melnig, Alcoholic reduction platinum nanoparticles synthesis by sonochemical method, Biomater. Biophys., Medical Phys. Ecology, No. 11, 31–36 (2008).
D. Li and S. Komarneni, Synthesis of Pt nanoparticles and nanorods by microwave-assisted solvothermal technique, Z. Naturforch., 5, 1566–1572 (2006).
S. S. J. Mollaha, C. E. G. Henleyb, and S. R. P. Silvab, Photo-chemical synthesis of iron oxide nanowires induced by pulsed laser ablation of iron powder in liquid media, Integr. Ferroelectr.: An Int. J., 119, No. 1, 45–54 (2010).
D. O. Oseguera-Galindo, A. Martínez-Benítez, A. Chávez-Chávez, G. Gómez-Rosas, A. Pérez-Centeno, and M. A. Santana-Aranda, Effects of the confining solvent on the size distribution of silver NPs by laser ablation, J. Nanoparticle Res., 14, No. 9, 1133 (2012).
H. J. Hah, S. M. Koo, and S. H. Lee, Preparation of silver nanoparticles through alcohol reduction with organoalkoxysilanes, J. Sol-Gel Sci. Technol., 26, 467–471 (2003).
C. Gong, M. Acik, R. M. Abolfath, Y. Chabal, and K. Cho, Graphitization of graphene oxide with ethanol during thermal reduction, J. Phys. Chem. C, 116, No. 18, 9969–9979 (2012).
P. Banerjee, S. Chakrabarti, S. Maitra, and B. K. Dutta, Zinc oxide nanoparticles – Sonochemical synthesis, characterization and application for photo-remediation of heavy metal, Ultrason. Sonochem., 19, No. 1, 85–93 (2012).
B. Liu, Y. Wang, Y. Huang, P. Dong, and S. Yin, Morphological control and photocatalytic activities of nitrogen-doped titania nanoparticles by microwave-assisted solvothermal process, J. Aust. Ceram. Soc., 48, 249–252 (2012).
D. Liang, C. Cui, H. Hu, Y. Wang, S. Xu, B. Ying, P. Li, B. Lu, and H. Shen, One-step hydrothermal synthesis of anatase TiO2/reduced graphene oxide nanocomposites with enhanced photocatalytic activity, J. Alloys Compd., Issue 582, 236–240 (2014).
T. Nakamura, Y. Tsukahara, T. Sakata, H. Mori, Y. Kanbe, H. Bessho, and Y. Wada, Preparation of monodispersed Cu nanoparticles by microwave-assisted alcohol reduction, Bull. Chem. Soc. Jpn., 80, 1492–1503 (2007).
Y. Hattori, S. Mukasa, H. Toyota, T. Inoue, and S. Nomura, Synthesis of zinc and zinc oxide nanoparticles from zinc electrode using plasma in liquid, Mater. Lett., 65, No. 2, 188–190 (2011).
N. Amaliyah, S. Mukasa, S. Nomura, and H. Toyota, Plasma in-liquid method for reduction of zinc oxide in zinc nanoparticle synthesis, Mater. Res. Express, 2, No. 2, 025004 (2015).
Y. Hattori, S. Nomura, S. Mukasa, H. Toyota, T. Inoue, and T. Kasahara, Synthesis of tungsten trioxide nanoparticles by microwave plasma in-liquid and analysis of physical properties, J. Alloys Compd., Issue 560, 105–110 (2013).
C. Qi-Yuan, Thermodynamic analysis of silicothermic reduction zinc oxide in vacuum, Acta Phys.-Chim. Sin., 27, No. 6, 1312–1318 (2011).
G. Gonzalez, S. Jordens, and S. Escobedo, Dependence of zinc oxide reduction rate on the CO concentration in CO/CO2 mixtures, Thermochim. Acta, 278, 129–134 (1996).
B.-S. Kim, J.-M. Yoo, J.-T. Park, and J.-C. Lee, A kinetic study of the carbothermic reduction of zinc oxide with various additives, Mater. Transact., 47, No. 9, 2421–2426 (2006).
C. Wieckert and A. Steinfeld, Solar thermal reduction of ZnO using CH4 : ZnO and C : ZnO molar ratios less than 1, J. Solar Energy Eng., 124, No. 1, 55–62 (2002).
H. A. Ebrahim and E. Jamshidi, Kinetic study of zinc oxide reduction by methane, Chem. Eng. Res. Des., 79, No. 1, 62–70 (2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Inzhenerno-Fizicheskii Zhurnal, Vol. 94, No. 6, pp. 1501–1506, November–December, 2021.
Rights and permissions
About this article
Cite this article
Amaliyah, N., Rahim, I., Putra, A.E.E. et al. In-Liquid Plasma Recycling Method of Zinc Oxide Nanoparticles. J Eng Phys Thermophy 94, 1467–1472 (2021). https://doi.org/10.1007/s10891-021-02426-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10891-021-02426-2