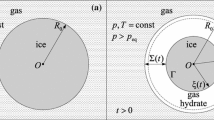
A diffusion model of dissociation of a plane layer of gas hydrate into ice and gas has been presented, which permits modeling the effect of self-preservation of gas hydrates. In this model, the gas-hydrate dissociation into ice and gas is described with account taken of the internal kinetics of the process and of the pore structure of the formed ice layer. Calculated data obtained within the framework of a quasi-stationary approximation for the cases of dissociation of plane layers of methane hydrate and carbon-dioxide hydrate into ice and gas have been given as an example.
Similar content being viewed by others
References
Z. R. Chong, S. H. B. Yang, P. Babu, P. Linga, and X.-S. Li, Review of natural gas hydrates as an energy resource: prospects and challenges, Appl. Energy, 162, 1633–1652 (2016).
Y. Konno, T. Fujii, A. Sato, K. Akamine, M. Naiki, Y. Masuda, K. Yamamoto, and J. Nagao, Key findings of the world’s first offshore methane hydrate production test off the coast of Japan: toward future commercial production, Energy Fuels, 31, No. 3, 2607–2616 (2017).
G. G. Tsypkin, Formation of carbon dioxide hydrate at the injection of carbon dioxide into a depleted hydrocarbon field, Fluid Dyn., 49, No. 6, 789–795 (2014).
V. Sh. Shagapov, N. G. Musakaev, and M. K. Khasanov, Formation of gas hydrates in a porous medium during an injection of cold gas, Int. J. Heat Mass Transf., 84, 1030–1039 (2015).
M. K. Khasanov, Investigation of regimes of gas hydrate formation in a porous medium, partially saturated with ice, Thermophys. Aeromech., 22, No. 2, 245–255 (2015).
É. A. Bondarev, I. I. Rozhin, V. V. Popov, and K. K. Argunova, Assessing the possibility of underground storage of natural-gas hydrates in the permafrost zone, Kriosfera Zemli, 19, No. 4, 64–74 (2015).
V. M. Vorotyntsev, V. M. Malyshev, I. V. Vorotyntsev, and S. V. Battalov, Improving the efficiency of gas hydrate crystallization due to the application of gas separation membranes, Theor. Found. Chem. Eng., 50, No. 4, 459–468 (2016).
M. K. Khasanov and V. Sh. Shagapov, Methane gas hydrate decomposition in a porous medium upon injection of a warm carbon dioxide gas, J. Eng. Phys. Thermophys., 89, No. 5, 1123–1133 (2016).
V. Sh. Shagapov, A. S. Chiglintseva, and S. V. Belova, On the theory of formation of a gas hydrate in a heat-insulated space compacted with membrane, J. Eng. Phys. Thermophys., 90, No. 5, 1147–1161 (2017).
G. Rehder, R. Eckl, M. Elfgen, A. Falenty, R. Hamann, N. Kähler, W. F. Kuhs, H. Osterkamp, and C. Windmeier, Methane hydrate pellet transport using the self-preservation effect: a techno-economic analysis, Energies, 5, No. 7, 2499–2523 (2012).
A. Falenty, W. F. Kuhs, M. Glockzin, and G. Rehder, "Self-preservation" of CH4 hydrates for gas transport technology: Pressure–temperature dependence and ice microstructures, Energy Fuels, 28, No. 10, 6275–6283 (2014).
H. Mimachi, S. Takeya, A. Yoneyama, K. Hyodo, T. Takeda, Y. Gotoh, and T. Murayama, Natural gas storage and transportation within gas hydrate of smaller particle: Size dependence of self-preservation phenomenon of natural gas hydrate, Chem. Eng. Sci., 118, 208–213 (2014).
H. P. Veluswamy, A. Kumar, Y. Seo, J. D. Lee, and P. Linga, A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates, Appl. Energy, 216, 262–285 (2018).
V. A. Istomin, V. S. Yakushev, N. A. Makhonina, V. G. Kwon, and E. M. Chuvilin, Self-preservation phenomenon of gas hydrates, Gas Ind. Russ., No. 4, 16–27 (2006).
O. S. Subbotin, V. R. Belosludov, E. N. Brodskaya, E. M. Piotrovskaya, and V. V. Sizov, A computer simulation of the mechanism of self-conservation of gas hydrates, Russ. J. Phys. Chem. A, 82, No. 8, 1303–1308 (2008).
A. Falenty and W. F. Kuhs, "Self-preservation" of CO2 gas hydrates–surface microstructure and ice perfection, J. Phys. Chem. B, 113, No. 49, 15975–15988 (2009).
H. Ohno, O. Nishimura, K. Suzuki, H. Narita, and J. Nagao, Morphological and compositional characterization of self-preserved gas hydrates by low-vacuum scanning electron microscopy, Phys. Chem. Chem. Phys., 12, No. 9, 1661–1665 (2011).
D. Sun, Y. Shimono, S. Takeya, and R. Ohmura, Preservation of carbon dioxide clathrate hydrate at temperatures below the water freezing point under atmospheric pressure, Ind. Eng. Chem. Res., 50, No. 24, 13854–13858 (2011).
A. Hachikubo, S. Takeya, E. Chuvilin, and V. Istomin, Preservation phenomena of methane hydrate in pore spaces, Phys. Chem. Chem. Phys., 13, No. 39, 17449–17452 (2011).
V. E. Nakoryakov and S. Ya. Misyura, The features of self-preservation for hydrate systems with methane, Chem. Eng. Sci., 104, 1–9 (2013).
A. S. Stoporev, A. Yu. Manakov, L. K. Altunina, A. V. Bogoslovsky, L. A. Strelets, and E. Ya. Aladko, Unusual self-preservation of methane hydrate in oil suspensions, Energy Fuels, 28, No. 2, 794–802 (2014).
V. E. Nakoryakov and S. Ya. Misyura, Kinetics of methane hydrate dissociation, Dokl. Phys. Chem., 464, No. 2, 244–246 (2015).
V. P. Mel’nikov, L. S. Podenko, A. N. Nesterov, A. O. Drachuk, N. S. Molokitina, and A. M. Reshetnikov, Self-preservation of methane hydrates produced in "dry water," Dokl. Chem., 466, No. 2, 53–56 (2016).
S. Takeya, S. Muromachi, Y. Yamamoto, H. Umeda, and S. Matsuo, Preservation of CO2 hydrate under different atmospheric conditions, Fluid Phase Equilibria, 413, 137–141 (2016).
É. D. Ershov, Yu. P. Lebedenko, E. M. Chuvilin, V. A. Istomin, and V. S. Yakushev, Features of the existence of gas hydrates in the cryolithic zone, Dokl. Akad. Nauk SSSR, 321, No. 4, 788–791 (1991).
V. S. Yakushev, E. V. Perlova, and N. A. Makhonina, Metastable (relict) gas hydrates: occurrence, resources, and prospects for utilization, Kriosfera Zemli, 9, No. 1, 68–72 (2005).
V. Sh. Shagapov and B. I. Tazetdinov, On the theory of the decomposition of a metastable gas hydrate, Theor. Found. Chem. Eng., 47, No. 4, 388–396 (2013).
E. P. Zaporozhets and N. A. Shostak, Adsorption-energy model of the kinetics of the formation and dissociation of gas hydrates, Theor. Found. Chem. Eng., 49, No. 3, 306–312 (2015).
T. Komai, S.-P. Kang, J.-H. Yoon, Y. Yamamoto, T. Kawamura, and M. Ohtake, In situ Raman spectroscopy investigation of the dissociation of methane hydrate at temperatures just below the ice point, J. Phys. Chem. B, 108, No. 23, 8062–8068 (2004).
C.-Y. Sun and G.-J. Chen, Methane hydrate dissociation above 0oC and below 0oC, Fluid Phase Equilibria, 242, No. 2, 123–128 (2006).
V. A. Vlasov, Diffusion model of gas hydrate dissociation into ice and gas: simulation of the self-preservation effect, Int. J. Heat Mass Transf., 102, 631–636 (2016).
V. A. Vlasov, Phenomenological diffusion theory of formation of gas hydrate from ice powder, Theor. Found. Chem. Eng., 46, No. 6, 576–582 (2012).
V. A. Vlasov, Formation and dissociation of gas hydrate in terms of chemical kinetics, React. Kinet. Mech. Catal., 110, No. 1, 5–13 (2013).
V. A. Vlasov, Diffusion model of gas hydrate formation from ice, Heat Mass Transf., 52, No. 3, 531–537 (2016).
V. Sh. Shagapov, A. S. Chiglintseva, and G. R. Rafikova, On quasistationary solution of the equation of gas diffusion in hydrate layer, Tomsk State Univ. J. Math. Mech., No. 48, 107–117 (2017).
L. A. Stern, S. Circone, S. H. Kirby, and W. B. Durham, Anomalous preservation of pure methane hydrate at 1 atm, J. Phys. Chem. B., 105, No. 9, 1756–1762 (2001).
W. F. Kuhs, G. Genov, D. K. Staykova, and T. Hansen, Ice perfection and onset of anomalous preservation of gas hydrates, Phys. Chem. Chem. Phys., 6, No. 21, 4917–4920 (2004).
S. Circone, L. A. Stern, S. H. Kirby, W. B. Durham, B. C. Chakoumakos, C. J. Rawn, A. J. Rondinone, and Y. Ishii, CO2 hydrate: synthesis, composition, structure, dissociation behavior, and a comparison to structure I CH4 hydrate, J. Phys. Chem. B, 107, No. 23, 5529–5539 (2003).
T. Ikeda-Fukazawa, K. Kawamura, and T. Hondoh, Mechanism of molecular diffusion in ice crystals, Mol. Simul., 30, Nos. 13–15, 973–979 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Inzhenerno-Fizicheskii Zhurnal, Vol. 92, No. 6, pp. 2449–2457, November–December, 2019.
Rights and permissions
About this article
Cite this article
Vlasov, V.A. Mathematical Model of the Effect of Self-Preservation of Gas Hydrates. J Eng Phys Thermophy 92, 1406–1414 (2019). https://doi.org/10.1007/s10891-019-02057-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10891-019-02057-8