Abstract
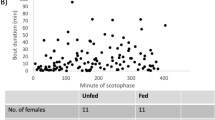
Most species of moths use a female-produced sex pheromone to bring mates together. Typically, sex pheromone is synthesized in a specialized gland and released during the behavior of “calling”, in which the ovipositor and gland are extruded, allowing pheromone to evaporate. Although there has been much study on how a gland makes specific pheromone components, we know relatively little about how it actually functions with regard to synthesis, storage and release. In this paper, we investigated three aspects of gland function in the noctuid moth Chloridea virescens (Fabricius): (i) whether translocation of pheromone from site of synthesis to release is dependent on calling or ovipositor movement, (ii) whether pheromone synthesis rate limits release and (iii) how intermittent calling (observed in this and other species) might affect the dynamics of release rate. Firstly, by manipulating the gland to simulate calling (extruded) or non-calling (retracted), we showed that pheromone translocation occurred regardless of whether the gland was retracted or extruded. Secondly, by manipulating pheromone production, we found that females that produced more pheromone had higher release rates. It was especially noticeable that females had a higher release rate at the start of calling, which dropped rapidly and leveled off over time. Together, these data suggest that intermittent calling in C. virescens (and other species) may function to allow females to replenish pheromone stores on the gland surface between calling bouts, so that brief, high release rates occur at the start of a calling bout; thus, potentially increasing a female’s chances of attracting a mate.



Similar content being viewed by others
References
Allison JD, Cardé RT (2016) Variation in moth pheromone: causes and consequences. In: Allison JD, Cardé RT (eds) Pheromone communication in moths: evolution, behavior and application. University of California Press, Oakland, pp 25–41
Almeida ÂA, Lima ER, Reis R Jr (2008) Pupal period affects calling behavior of the wheat moth, Pseudaletia sequax (Lepidoptera: Noctuidae). Ethology 114:499–503. https://doi.org/10.1111/j.1439-0310.2008.01492.x
Bjostad LB, Linn CE, Du JW, Roelofs WL (1984) Identification of new sex pheromone components in Trichoplusia ni, predicted from biosynthetic precursors. J Chem Ecol 10:1309–1323
Cardé RT (2016) Moth navigation along pheromone plumes. In: Allison JD, Cardé RT (eds) Pheromone communication in moths: evolution, behavior and application. University of California Press, Berkeley, pp 173–189
Conner WE, Webster RP, Itagaki H (1985) Calling behaviour in arctiid moths: the effects of temperature and wind speed on the rhythmic exposure of the sex attractant gland. J Insect Physiol 31:815–820. https://doi.org/10.1016/0022-1910(85)90074-5
Eltahlawy H, Buckner JS, Foster SP (2007) Evidence for two-step regulation of pheromone biosynthesis by the pheromone biosynthesis-activating neuropeptide in the moth Heliothis virescens. Arch Insect Biochem Physiol 64:120–130
Foster SP (2016) Toward a quantitative paradigm for sex pheromone production in moths. In: Allison JD, Cardé RT (eds) Pheromone communication in moths: evolution, behavior and application. University of California Press, Oakland, pp 113–126
Foster S, Anderson K (2011) The use of mass isotopomer distribution analysis to quantify synthetic rates of sex pheromone in the moth Heliothis virescens. J Chem Ecol 37:1208–1210
Foster SP, Anderson KG (2015) Sex pheromones in mate assessment: analysis of nutrient cost of sex pheromone production by females of the moth Heliothis virescens. J Exp Biol 218:1252–1258. https://doi.org/10.1242/jeb.119883
Foster SP, Anderson KG (2018) Differential pheromone sampling of the gland of female Heliothis virescens moths reveals gandular differences in composition and quantity. J Chem Ecol 44:452–462. https://doi.org/10.1007/s10886-018-0954-0
Foster SP, Anderson KG, Casas J (2018) The dynamics of pheromone gland synthesis and release: a paradigm shift for understanding sex pheromone quantity in female moths. J Chem Ecol 44:525–533. https://doi.org/10.1007/s10886-018-0963-z
Foster SP, Anderson KG, Casas J (2020) Calling behavior and sex pheromone release and storage in the moth Chloridea virescens. J Chem Ecol 46:10–20. https://doi.org/10.1007/s10886-019-01133-w
Frérot B, Malosse C, Cain A-H (1997) Solid-phase microextraction (SPME): a new tool in pheromone identification in Lepidoptera. J High Resolut Chromatogr 20:340–342
Groot AT (2014) Circadian rhythms of sexual activities in moths: a review. Front Ecol Evol 2:43. https://doi.org/10.3389/fevo.2014.00043
Groot AT, Nojima S, Heath JJ, Ammagarahalli B, van Wijk M, Claβen A, Santangelo RG, Lopez J, Schal C (2018) Alcohol contributes to attraction of Heliothis (= Chloridea) virescens males to females. J Chem Ecol 44:621–630. https://doi.org/10.1007/s10886-018-0995-4
Hagström ÅK, Walther A, Wendland J, Löfstedt C (2013) Subcellular localization of the fatty acyl reductase involved in pheromone biosynthesis in the tobacco budworm, Heliothis virescens (Noctuidae: Lepidoptera). Insect Biochem Mol Biol 43:510–521. https://doi.org/10.1016/j.ibmb.2013.03.006
Heath RR, McLaughlin JR, Proshold F, Teal PEA (1991) Periodicity of female sex pheromone titer and release in Heliothis subflexa and H. virescens (Lepidoptera: Noctuidae). Ann Entomol Soc Am 84:182–189
Jurenka RA, Roelofs WL (1989) Characterization of the acetyltransferase used in pheromone biosynthesis in moths: specificity for the Z isomer in Tortricidae. Insect Biochem 19:639–644
Klun JA, Bierl-Leonhardt BA, Plimmer JR, Sparks AN, Primiani M, Chapman OL, Lepone G, Lee GH (1980) Sex pheromone chemistry of the female tobacco budworm moth, Heliothis virescens. J Chem Ecol 6:177–184
Lievers R, Groot AT (2016) Disposable Polydimethylsiloxane (PDMS)-coated fused silica optical fibers for sampling pheromones of moths. PLoS One 11:e0161138
Ma PWK, Ramaswamy SB (2003) Biology and ultrastructure of sex pheromone-producing tissue. In: Blomquist GJ, Vogt RC (eds) Insect pheromone biochemsitry and molecular biology. Elsevier Academic Press, London, pp 19–51
Nojima S, Classen A, Groot AT, Schal C (2018) Qualitative and quantitative analysis of chemicals emitted from the pheromone gland of individual Heliothis subflexa females. PLoS One 13:e0202035. https://doi.org/10.1371/journal.pone.0202035
Raina AK, Wergin WP, Murphy CA, Erbe EF (2000) Structural organization of the sex pheromone gland in Helicoverpa zea in relation to pheromone production and release. Arthropod Struct Dev 29:343–353
Ramaswamy SB (1990) Periodicity of oviposition, feeding, and calling by mated female Heliothis virescens in a field cage. J Insect Behav 3:417–427. https://doi.org/10.1007/BF01052118
Schal C, Cardé RT (1985) Rhythmic extrusion of pheromone gland elevates pheromone release rate. Experientia 41:1617–1619
Swier SR, Rings RW, Musick GJ (1977) Age-related calling behavior of the black cutworm, Agrotis ipsilon. Ann Entomol Soc Am 70:919–924. https://doi.org/10.1093/aesa/70.6.919
Symonds MRE, Johnson TL, Elgar MA (2012) Pheromone production, male abundance, body size, and the evolution of elaborate antennae in moths. Ecol Evol 2:227–246. https://doi.org/10.1002/ece3.81
Tang JD, Charlton RE, Jurenka RA, Wolf WA, Phelan PL, Sreng L, Roelofs WL (1989) Regulation of pheromone biosynthesis by a brain hormone in two moth species. Proc Natl Acad Sci U S A 86:1806–1810
Teal PEA, Tumlinson JH (1987) The role of alcohols in pheromone biosynthesis by two noctuid moths that use acetate pheromone components. Arch Insect Biochem Physiol 4:261–269. https://doi.org/10.1002/arch.940040404
Teal PEA, Tumlinson JH (1988) Properties of cuticular oxidases used for sex pheromone biosynthesis by Heliothis zea. J Chem Ecol 14:2131–2145
Umbers KDL, Symonds MRE, Kokko H (2015) The mothematics of female pheromone signaling: strategies for aging virgins. Am Nat 185:417–432. https://doi.org/10.1086/679614
Zhao JZ, Haynes KF (1997) Does PBAN play an alternative role of controlling pheromone emission in the cabbage looper moth, Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae)? J Insect Physiol 43:695–700. https://doi.org/10.1016/s0022-1910(97)00003-6
Acknowledgments
Funding supporting this work was provided by United States Department of Agriculture Hatch Project ND02388. We are grateful to the United States Department of Agriculture–National Institute of Food and Agriculture for an Instrument Grant, 2015-07238 contributing, in part, to the purchase of the GC/MS system.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Foster, S.P., Anderson, K.G. The Effect of Pheromone Synthesis and Gland Retraction on Translocation and Dynamics of Pheromone Release in the Moth Chloridea virescens. J Chem Ecol 46, 581–589 (2020). https://doi.org/10.1007/s10886-020-01198-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-020-01198-y