Abstract
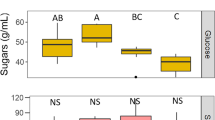
Microbial metabolism can shape cues important for animal attraction in service-resource mutualisms. Resources are frequently colonized by microbial communities, but experimental assessment of animal-microbial interactions often focus on microbial monocultures. Such an approach likely fails to predict effects of microbial assemblages, as microbe-microbe interactions may affect in a non-additive manner microbial metabolism and resulting chemosensory cues. Here, we compared effects of microbial mono- and cocultures on growth of constituent microbes, volatile metabolite production, sugar catabolism, and effects on pollinator foraging across two nectar environments that differed in sugar concentration. Growth in co-culture decreased the abundance of the yeast Metschnikowia reukaufii, but not the bacterium Asaia astilbes. Volatile emissions differed significantly between microbial treatments and with nectar concentration, while sugar concentration was relatively similar among mono- and cocultures. Coculture volatile emission closely resembled an additive combination of monoculture volatiles. Despite differences in microbial growth and chemosensory cues, honey bee feeding did not differ between microbial monocultures and assemblages. Taken together, our results suggest that in some cases, chemical and ecological effects of microbial assemblages are largely predictable from those of component species, but caution that more work is necessary to predict under what circumstances non-additive effects are important.





Similar content being viewed by others
References
Allard SM, Ottesen AR, Brown EW, Micallef SA (2018) Insect exclusion limits variation in bacterial microbiomes of tomato flowers and fruit. J Appl Microbiol 125:1749–1760. https://doi.org/10.1111/jam.14087
Álvarez-Pérez S, Herrera CM (2013) Composition, richness and nonrandom assembly of culturable bacterial-microfungal communities in floral nectar of Mediterranean plants. FEMS Microbiol Ecol 83:685–699. https://doi.org/10.1111/1574-6941.12027
Álvarez-Pérez S, Herrera CM, de Vega C (2012) Zooming-in on floral nectar: A first exploration of nectar-associated bacteria in wild plant communities. FEMS Microbiol Ecol 80:591–602. https://doi.org/10.1111/j.1574-6941.2012.01329.x
Álvarez-Pérez S, Lievens B, Fukami T (2019) Yeast-bacterium interactions: the next frontier in nectar research. Trends Plant Sci.https://doi.org/10.1016/j.tplants.2019.01.012
Bascompte J, Jordano P (2007) Plant-animal mutualistic networks: The architecture of biodiversity. Annu Rev Ecol Evol S 38:567–593. https://doi.org/10.1146/annurev.ecolsys.38.091206.095818
Bauer MA, Kainz K, Carmona-Gutierrez D, Madeo F (2018) Microbial wars: Competition in ecological niches and within the microbiome. Microb Cell 5:215–219. https://doi.org/10.15698/mic2018.05.628
Beisel CL, Afroz T (2016) Rethinking the Hierarchy of Sugar Utilization in Bacteria. J Bacteriol 198:374–376. https://doi.org/10.1128/jb.00890-15
Bronstein JL (2015) The study of mutualism. In: Bronstein JL (ed) Mutualism. Oxford University Press, New York, p 3–19
Buchholz R, Levey DJ (1990) The evolutionary triad of microbes, fruits, and seed dispersers - an experiment in fruit choice by cedar waxwings, Bombycilla cedrorum. Oikos 59:200–204. https://doi.org/10.2307/3545535
Crotti E et al (2010) Acetic acid bacteria, newly emerging symbionts of insects. Appl Environ Microbiol 76:6963–6970. https://doi.org/10.1128/aem.01336-10
Davis TS, Crippen TL, Hofstetter RW, Tomberlin JK (2013) Microbial volatile emissions as insect semiochemicals. J Chem Ecol 39:840–859. https://doi.org/10.1007/s10886-013-0306-z
Dhami MK, Hartwig T, Fukami T (2016) Genetic basis of priority effects: insights from nectar yeast. Proc R Soc B 283. https://doi.org/10.1098/rspb.2016.1455
Fischer C, Trautman EP, Crawford JM, Stabb EV, Handelsman J, Broderick NA (2017) Metabolite exchange between microbiome members produces compunds that influence Drosophila behavior. eLife 6 doi:https://doi.org/10.7554/eLife.18855
Fridman S, Izhaki I, Gerchman Y, Halpern M (2012) Bacterial communities in floral nectar. Environ Microbiol Rep 4:97–104. https://doi.org/10.1111/j.1758-2229.2011.00309.x
Good AP, Gauthier M-PL, Vannette RL, Fukami T (2014) Honey bees avoid nectar colonized by three bacterial species, but not by a yeast species, isolated from the bee gut. PLoS One 9:e86494
Granato ET, Meiller-Legrand TA, Foster KR (2019) The evolution and ecology of bacterial warfare. Curr Biol 29:R521–R537. doi:https://doi.org/10.1016/j.cub.2019.04.024
Herrera CM, Garcia IM, Perez R (2008) Invisible floral larcenies: Microbial communities degrade floral nectar of bumble bee-pollinated plants. Ecology 89:2369–2376. doi:https://doi.org/10.1890/08-0241.1
Herrera CM, Canto A, Pozo MI, Bazaga P (2009a) Inhospitable sweetness: nectar filtering of pollinator-borne inocula leads to impoverished, phylogenetically clustered yeast communities. P Roy Soc B-Biol Sci 277:747–754. doi:10.1098/rspb.2009a.1485
Herrera CM, de Vega C, Canto A, Pozo MI (2009b) Yeasts in floral nectar: A quantitative survey. Ann Bot 103:1415–1423. doi:10.1093/aob/mcp026
Herrera CM, Pozo MI, Medrano M (2013) Yeasts in nectar of an early-blooming herb: Sought by bumble bees, detrimental to plant fecundity. Ecology 94:273–279
Jakobsen HB, Kristjansson K, Rohde B, Terkildsen M, Olsen CE (1995) Can social bees be influenced to choose a specific feeding station by adding the scent of the station to the hive air? J Chem Ecol 21:1635–1648. https://doi.org/10.1007/bf02033666
Junker RR, Romeike T, Keller A, Langen D (2014) Density-dependent negative responses by bumblebees to bacteria isolated from flowers. Apidologie 45:467–477. https://doi.org/10.1007/s13592-013-0262-1
Kešnerová L, Emery O, Troilo M, Liberti J, Erkosar B, Engel P (2019) Gut microbiota structure differs between honeybees in winter and summer. ISME J.https://doi.org/10.1038/s41396-019-0568-8
Lachance MA, Starmer WT, Rosa CA, Bowles JM, Barker JSF, Janzen DH (2001) Biogeography of the yeasts of ephemeral flowers and their insects. FEMS Yeast Res 1:1–8
Lawson DA, Rands SA (2019) The effects of rainfall on plant-pollinator interactions. Arthropod-Plant Inte 13:561–569. https://doi.org/10.1007/s11829-019-09686-z
Lemfack MC, Nickel J, Dunkel M, Preissner R, Piechulla B (2014) mVOC: A database of microbial volatiles. Nucleic Acids Res 42:D744–D748. https://doi.org/10.1093/nar/gkt1250
Lenaerts M, Pozo MI, Wäckers F, Van den Ende W, Jacquemyn H, Lievens B (2016) Impact of microbial communities on floral nectar chemistry: Potential implications for biological control of pest insects . Basic Appl Ecol 17:189–198. https://doi.org/10.1016/j.baae.2015.10.001
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15. https://doi.org/10.1186/s13059-014-0550-8
Martinez-Buitrago PA, Ramos FA, Castellano L (2019) Binary co-culture selection from marine-derived microorganisms for differential production of specialized metabolites. Quim Nova 42:713–719. https://doi.org/10.21577/0100-4042.20170388
Menzel R (1993) Associative learning in honey bees. Apidologie 24:157–168. doi:https://doi.org/10.1051/apido:19930301
Mittelbach M, Yurkov AM, Stoll R, Begerow D (2016) Inoculation order of nectar-borne yeasts opens a door for transient species and changes nectar rewarded to pollinators. Fungal Ecol 22:90–97. doi:https://doi.org/10.1016/j.funeco.2015.12.003
Mommaerts V, Wäckers F, Smagghe G (2013) Assessment of gustatory responses to different sugars in harnessed and free-moving bumblebee workers (Bombus terrestris). Chem Senses 38:399–407. https://doi.org/10.1093/chemse/bjt014
Morris BE, Henneberger R, Huber H, Moissl-Eichinger C (2013) Microbial syntrophy: Interaction for the common good . FEMS Microbiol Rev 37:384–406. https://doi.org/10.1111/1574-6976.12019
Oksanen J et al (2018) vegan: Community Ecology Package. R package version 2.5-2. https://CRAN.R-project.org/package=vegan
Pamminger T, Becker R, Himmelreich S, Schneider CW, Bergtold M (2019) The nectar report: quantitative review of nectar sugar concentrations offered by bee visited flowers in agricultural and non-agricultural landscapes. PeerJ 7. https://doi.org/10.7717/peerj.6329
Peay KG, Belisle M, Fukami T (2012) Phylogenetic relatedness predicts priority effects in nectar yeast communities. Proc R Soc B 279:749–758. https://doi.org/10.1098/rspb.2011.1230
Peris JE, Rodriguez A, Pena L, Fedriani JM (2017) Fungal infestation boosts fruit aroma and fruit removal by mammals and birds. Sci Rep 7. https://doi.org/10.1038/s41598-017-05643-z
Pinheiro J, Bates D, DebRoy S, Sarkar D, Core Team R (2019) nlme: Linear and nonlinear mixed effects models. R package version 3.1–139. https://CRAN.R-project.org/package=nlme
Ponomarova O, Patil KR (2015) Metabolic interactions in microbial communities: Untangling the Gordian knot. Curr Opin Microbiol 27:37–44. https://doi.org/10.1016/j.mib.2015.06.014
Pozo MI et al (2019) The impact of yeast presence in nectar on bumble bee behavior and fitness. Ecol Monogr.https://doi.org/10.1002/ecm.1393
Pozo MI, Herrera CM, Bazaga P (2011) Species richness of yeast communities in floral nectar of southern spanish plants. Microb Ecol 61:82–91. https://doi.org/10.1007/s00248-010-9682-x
Pozo MI, Lievens B, Jacquemyn H (2015) Impact of microorganisms on nectar chemistry, pollinator attraction and plant fitness. In: Peck RL (ed) Nectar: Production, Chemical Composition and Benefits to Animals and Plants. Nova Publishers, New York, pp 1–45
Raguso RA (2004) Why are some floral nectars scented? Ecology 85:1486–1494. https://doi.org/10.1890/03-0410
Rering CC, Beck JJ, Hall GW, McCartney MM, Vannette RL (2018) Nectar-inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator. New Phytol 220:750–759. doi:https://doi.org/10.1111/nph.14809
Ruxton GD, Wilkinson DM, Schaefer HM, Sherratt TN (2014) Why fruit rots: Theoretical support for Janzen’s theory of microbe-macrobe competition. Proc R Soc B 281. https://doi.org/10.1098/rspb.2013.3320
Schaeffer RN, Vannette RL, Irwin RE (2015) Nectar yeasts in Delphinium nuttallianum (Ranunculaceae) and their effects on nectar quality. Fungal Ecol 18:100–106. https://doi.org/10.1016/j.funeco.2015.09.010
Schaeffer RN, Mei YZ, Andicoechea J, Manson JS, Irwin RE (2017) Consequences of a nectar yeast for pollinator preference and performance. Funct Ecol 31:613–621. doi:https://doi.org/10.1111/1365-2435.12762
Schaeffer RN, Rering CC, Maalouf I, Beck JJ, Vannette RL (2019) Microbial metabolites mediate bumble bee attraction and feeding. Biol Lett 15:20190132
Schwarzenbach RP, Gschwend PM, Imboden DM (2003) Environmental Organic Chemistry. Wiley, Hoboken
Seo HJ, Song J, Yoon HJ, Lee KY (2019) Effects of nectar contents on the foraging activity of honeybee (Apis mellifera) on Asian pear (Pyrus pyrifolia Nakai). Sci Hortic 245:185–192. doi:https://doi.org/10.1016/j.scienta.2018.10.009
Seth EC, Taga ME (2014) Nutrient cross-feeding in the microbial world. Front Microbiol 5. https://doi.org/10.3389/fmicb.2014.00350
Suzuki R, Zhang Y, Iino T, Kosako Y, Komagata K, Uchimura T (2010) Asaia astilbes sp nov., Asaia platycodi sp nov., and Asaia prunellae sp nov., novel acetic acid bacteria isolated from flowers in Japan. J Gen Appl Microbiol 56:339–346. https://doi.org/10.2323/jgam.56.339
Tang B-L et al (2020) A predator-prey interaction between a marine Pseudoalteromonas sp. and Gram-positive bacteria. Nat Commun 11:285. doi:https://doi.org/10.1038/s41467-019-14133-x
Toju H, Vannette RL, Gauthier MPL, Dhami MK, Fukami T (2018) Priority effects can persist across floral generations in nectar microbial metacommunities. Oikos 127:345–352. https://doi.org/10.1111/oik.04243
Tsuji K, Fukami T (2018) Community-wide consequences of sexual dimorphism: evidence from nectar microbes in dioecious plants. Ecology 99:2476–2484. https://doi.org/10.1002/ecy.2494
Tucker CM, Fukami T (2014) Environmental variability counteracts priority effects to facilitate species coexistence: evidence from nectar microbes. Proc R Soc B 281. https://doi.org/10.1098/rspb.2013.2637
Vander Wall SB (2001) The evolutionary ecology of nut dispersal. Bot Rev 67:74–117. https://doi.org/10.1007/bf02857850
Vannette RL, Fukami T (2017) Dispersal enhances beta diversity in nectar microbes. Ecol Lett 20:901–910. https://doi.org/10.1111/ele.12787
Vannette RL, Fukami T (2018) Contrasting effects of yeasts and bacteria on floral nectar traits. Ann Bot 121:1343–1349. https://doi.org/10.1093/aob/mcy032
Vannette RL, Gauthier MPL, Fukami T (2013) Nectar bacteria, but not yeast, weaken a plant-pollinator mutualism. Proc R Soc B 280. https://doi.org/10.1098/rspb.2012.2601
Verginer M, Leitner E, Berg G (2010) Production of volatile metabolites by grape-associated microorganisms. J Agric Food Chem 58:8344–8350. https://doi.org/10.1021/jf100393w
Wakefield J, Hassan HM, Jaspars M, Ebel R, Rateb ME (2017) Dual induction of new microbial secondary metabolites by fungal bacterial co-cultivation. Front Microbiol 8. https://doi.org/10.3389/fmicb.2017.01284
Wassermann B, Muller H, Berg G (2019) An apple a day: Which bacteria do we eat with organic and conventional apples? Front Microbiol 10. https://doi.org/10.3389/fmicb.2019.01629
Wykes GR (1952) The preferences of honeybees for solutions of various sugars which occur in nectar. J Exp Biol 29:511–519
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Acknowledgments
We thank M. Anderson, M. Handy, and I. Maalouf for laboratory and field assistance. This research was supported by USDA-ARS Research Project 6036-22000-028 (JB and CR), and 2016 ARS Administrator Research Associate program (CR). RS acknowledges support from a USDA NIFA Education and Literacy Initiative Postdoctoral Fellowship (2017-67012-26104). RV was supported by the National Science Foundation (award numbers DEB1846266 and DEB1929516), USDA Multistate award NE1501, the Hellman Foundation and the University of California, Davis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 278 kb)
Rights and permissions
About this article
Cite this article
Rering, C.C., Vannette, R.L., Schaeffer, R.N. et al. Microbial Co-Occurrence in Floral Nectar Affects Metabolites and Attractiveness to a Generalist Pollinator. J Chem Ecol 46, 659–667 (2020). https://doi.org/10.1007/s10886-020-01169-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-020-01169-3