Abstract
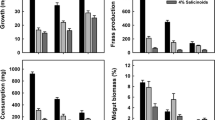
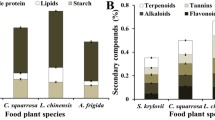
Generalist insect herbivores may regulate nutrient balance in their diets, including the incorporation of carbohydrates as well as proteins. However, secondary metabolites, including tannins, are likely to interact with dietary protein:carbohydrate ratios in insect herbivores. We investigated the effects of protein:carbohydrate ratios, tannin, and the interaction between macronutrient ratios and tannin on the performance of the gypsy moth Lymantria dispar. We designed a 6 X 3 factorial experiment, with six protein:carbohydrate ratios and three tannin concentrations. We monitored the development time and size of gypsy moths on the different diets. We conducted 4th stadium feeding trials to measure consumption, digestibility, and overall efficiency of ingestion/digestion. Gypsy moths fed a diet containing a 1:1 protein:carbohydrate ratio without tannin grew larger and developed faster than those fed a 1:2 protein:carbohydrate ratio diet. Increasing protein in the diet above the 1:1 protein:carbohydrate ratio (i.e. 2:1 or 7:1) did not have a significant effect on gypsy moth growth or development. Approximate digestibility was greatest in treatments with a low protein:carbohydrate ratio (1:2). Gypsy moths grew faster and larger on no-tannin diets than those with tannin in the diet. However, the specific concentration of tannin did not affect growth. The resulting interaction between protein:carbohydrate ratio and tannin showed that there may be a trade-off between development time and efficiency of food assimilation. We also found that feeding gypsy moth larvae an optimal protein:carbohydrate ratios may be more important for tolerating tannin than the amount of protein ingested alone.




Similar content being viewed by others
References
Agrawal A (2007) Macroevolution of plant defense strategies. Trends Ecol Evol 22:103–109
Altieri M, Letourneau D, Risch S (1984) Vegetation diversity and insect pest outbreaks. Crit Rev Plant Sci 2:131–169
Appel H, Maines L (1995) The influence of host plant on gut conditions of gypsy moth (Lymantria dispar) caterpillars. J Insect Physiol 41:241–246
Arnold TM, Schultz JC (2002) Induced sink strength as a prerequisite for induced tannin synthesis in developing leaves of Populus. Oecologia 130:585–593
Baldwin IT, Schultz JC (1983) Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science 221:277–279
Baldwin IT, Schultz JC, Ward D (1987) Patterns and sources of leaf tannin variation in yellow birch (Betula allegheniensis) and sugar maple (Acer saccharum). J Chem Ecol 13:1069–1078
Barbehenn R, Constabel CP (2011) Tannins in plant-herbivore interactions. Phytochemistry 72:1551–1565
Barbehenn R, Martin M (1992) The protective role of the peritrophic membrane in tannin tolerant larvae of Orgyia leucostigma (Lepidoptera). J Insect Physiol 38:973–980
Behmer S (2009) Insect herbivore nutrient regulation. Annu Rev Entomol 54:165–187
Berenbaum M (1983) Effects of tannins on growth and digestion in two species of papilinoids. Entomol Exp Appl 34:245–250
Bernays EA, Driver GC, Bilgener M (1989) Herbivores and plant tannins. In: Begon M, Fitter A, Ford E, Macfayden A (eds) Advances in ecological research vol 19. Elsevier, London, pp 263–302
Boeckler GA, Towns M, Unsicker SB, Mellway RD, Yip L, Hilke I, Gershenzon J, Constabel CP (2014) Transgenic upregulation of the condensed tannin pathway in poplar leads to a dramatic shift in leaf palatability for two tree-feeding Lepidoptera. J Chem Ecol 40:150–158
Chino H, Downer RH, Wyatt GR, Gilbert LI (1981) Lipophorins, a major class of lipoproteins of insect haemolymph. Insect Biochem 11:491
Clissold F, Sanson G, Read J (2006) The paradoxical effects of nutrient ratios and supply rates on an outbreaking insect herbivore, the Australian plague locust. J Anim Ecol 75:1000–1013
Coley PD (1983) Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol Monograph 53:209–234
Couture J, Mason C, Habeck C, Lindroth R (2016) Behavioral and morphological responses of an insect herbivore to low nutrient quality are inhibited by plant chemical defenses. Arthropod Plant Interact 10:341–349
Crawley MJ (1983) Herbivory: the dynamics of animal-plant interactions. Blackwell Scientific Publications, Oxford, U.K.
Davidson CB, Gottschalk KW, Johnson JE (1999) Tree mortality following defoliation by the European gypsy moth (Lymantria dispar L.) in the United States: a review. For Sci 45:74–84
Dawra RK, Makkar HPS, Singh B (1988) Total phenolics, condensed tannins, and protein-precipitable phenolics in young and mature leaves of oak species. J Agric Food Chem 36:951–953
De Veau EI, Schultz JC (1992) Reassessment of interaction between gut detergents and tannins in Lepidoptera and significance for gypsy moth larvae. J Chem Ecol 18:1437–1453
Elkinton J, Liebhold A (1990) Population dynamics of gypsy moth in North America. Annu Rev Entol 35:571–596
Elser J (2006) Biological stoichiometry: a chemical bridge between ecosystem ecology and evolutionary biology. Am Nat 168:25–35
Feeny P (1968) Effect of oak leaf tannins on larval growth of the winter moth Operophtera brumata. Insect Physiol 14:805–817
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581
Feeny P (1976) Plant Apparency and chemical defense. In: Wallace JW, Mansell RL (eds) Biochemical interaction between plants and insects. Springer, Boston, MA, pp 1–40
Feeny P, Bostock H (1968) Seasonal changes in the tannin content of oak leaves. Phytochemistry 7:871–880
Felton GW (1996) Nutritive quality of plant protein: sources of variation and insect herbivore responses. Arch Insect Biochem Physiol 32:107–130
Franco OL, Rigden DJ, Melo FR, Grossi-de-Sá MF (2002) Plant α-amylase inhibitors and their interaction with insect α-amylases. Eur J BioChem 269:397-412
Goncalves AZ, Mercier H, Oliveira RS, Romero GQ (2016) Trade-off between soluble protein production and nutritional storage in Bromeliaceae. Ann Bot 118:1199–1208
Grayson KL, Parry D, Faske TM, Hamilton A, Tobin PC, Agosta SI, Johnson DM (2015) Performance of wild and laboratory-reared gypsy moth (Lepidoptera: Erebidae): a comparison between foliage and artificial diet. Environ Entomol 44:864–873
Hafeez M, Liu S, Jan S, Gulzar A, Fernandez-Grandon GM, Qasim M et al (2019) Enhanced effects of dietary tannic acid with chlorantraniliprole on life table parameters and nutritional physiology of Spodoptera exigua. Pestic Biochem Physiol. https://doi.org/10.1013/j.pestbp.2019
Hagerman A (2011) Tannin Handbook. Miami University, Oxford, OH. https://www.users.miamioh.edu/hagermae/. Accessed 5 May 2019
Hemming JD, Lindroth RL (1995) Intraspecific variation in aspen phytochemistry: effects on performance of gypsy moths and forest tent caterpillars. Oecologia 103:79–88
IBM Corp (2016) IBM SPSS statistics for Windows, version 24.0. IBM Corp, Armonk, NY
Jankovic-Tomanic M, Lazarevic J (2012) Effects of temperature and dietary nitrogen on genetic variation and covariation in gypsy moth larval performance traits. Arch Biol Sci 64:1109–1116
Klocke J, Chan B (1982) Effects of cotton condensed tannin on feeding and digestion in the cotton pest, Heliothis zea. J Insect Physiol 28:911–915
Lazarevic J, Jankovic-Tomanic M, Savkovic U, Dordevic M, Milanovic S, Stojkovic B (2017) Host-associated divergence in the activity of digestive enzymes in two populations of the gypsy moth Lymantria dispar (Lepidoptera: Erebidae). Entomol Sci 20:189–194
Lee KP, Simpson SJ, Wilson K (2008) Dietary protein-quality influences melanization and immune function in an insect. Funct Ecol 22:1052–1061
Lemoine NP, Shantz AA (2016) Increased temperature causes protein limitation by reducing the efficiency of nitrogen digestion in the ectothermic herbivore Spodoptera exigua. Physiol Entomol 41:143–151
Liebhold A, Elkinton J, Williams D, Muzika RM (2000) What causes outbreaks of gypsy moth in North America? Popul Ecol 42:257–266
Lindroth RL, Bloomer MM (1991) Biochemical ecology of the forest tent caterpillar: responses to dietary protein and phenolic glycosides. Oecologia 86:408–413
Lindroth RL, Klein KA, Hemming JDC, Feuker AM (2008) Variation in temperature and dietary nitrogen affect performance of the gypsy moth (Lymantria dispar L.). Physiol Entomol 22:55–64
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609
Martin J, Martin M, Bernays E (1987) Failure of tannic acid to inhibit digestion or reduce digestibility of plant protein in gut fluids of insect herbivores. J Chem Ecol 13:605–621
Martin M, Martin J (1984) Surfactants: their role in preventing the precipitation of proteins by tannins in insect guts. Oecologia 61:342–345
Moise ER, McNeil JN, Hartley SE, Henry HA (2019) Plant silicon effects on insect feeding dynamics are influenced by plant nitrogen availability. Entomol Exp Appl 167:91–97
Muiruri EW, Barantal S, Iason G, Salminen JP, Perez-Fernandez E, Koricheva J (2019) Forest diversity effects on insect herbivores: do leaf traits matter? New Phytol 221:2250–2260
Muller C, Orians CM (2018) From plants to herbivores: novel insights into the ecological and evolutionary consequences of plant variation. Oecologia 187:357–360
Negreiros ANM, Carvalho MM, Xavier-Filho J, Blanco-Labra A, Shewry PR, Richardson M (1991) The complete amino acid sequence of the major Kunitz trypsin inhibitor from the seeds of Prosopsis juliflora. Phytochemistry 30:2829–2833
Peters DJ, Constabel CP (2002) Molecular analysis of herbivore-induced plant volatiles in maize. Planta 234:207–215
Pyke GH, Pulliam HR, Charnov DL (1977) Optimal foraging: a selective review of theory and tests. Q Rev Biol 52:137–154
Raubenheimer D, Simpson SJ (1993) The geometry of compensatory feeding in the locust. Anim Behav 45:953–964
Raubenheimer D, Simpson SJ (1994) The analysis of nutrient budgets. Funct Ecol 8:783–791
Raubenheimer D, Simpson SJ (1997) Integrative models of nutrient balancing: application to insects and vertebrates. Nutr Res 10:151–179
Rhoades DF, Cates RG (1976) Toward a general theory of plant antiherbivore chemistry. In: Wallace J, Mansell R (eds) Biochemical interaction between plants and insects. Springer, Boston, MA, pp 168–213
Rossiter M (1987) Genetic and phenotypic variation in diet breadth in a generalist herbivore. Evol Ecol 1:272–282
Rossiter M, Schultz JC, Baldwin IT (1988) Relationships among defoliation, red oak phenolics, and gypsy moth growth and reproduction. Ecology 69:267–277
Roth SK, Lindroth RL, Montgomery ME (1994) Effects of foliar phenolics and ascorbic acid on performance of the gypsy moth (Lymantria dispar). Biochem Syst Ecol 22:341–351
Schroeder LA (1986) Protein limitation of a tree leaf feeding lepidopteran. Entomol Exp Appl. 41:115–120
Schultz JC (1989) Tannin-insect interactions. In: Hemingway J, Karchesy J, Branham S (eds) Chemistry and significance of condensed tannins. Springer, Boston, MA, pp 417–433
Schultz JC, Baldwin IT (1982) Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217:149–151
Scriber JM (1977) Limiting effects of low leaf-water content on the nitrogen utilization, energy budget, and larval growth of Hyalophora cecropia (Lepidoptera: Saturniidae). Oecologia 28:269-287
Sharov AA, Leonard D, Liebhold AM, Roberts EA, Dickerson W (2002) "slow the spread": a national program to contain the gypsy moth. J For 100:30–36
Simpson SJ, Raubenheimer D (1993) Multi-level analysis of feeding behavior: geometry of nutritional decisions. Phil Trans R Soc Lond 342:381–402
Simpson SJ, Raubenheimer D (1995) The geometric analysis of feeding and nutrition: a user's guide. J Insect Physiol 7:545–553
Simpson SJ, Raubenheimer D (1999) Assuaging nutritional complexity: a geometric approach. Proc Nutri Soc 58:779–789
Simpson SJ, Raubenheimer D (2001) The geometric analysis of nutrient-allelochemical interactions: a case study using locusts. Ecology 82:422–439
Slansky F, Wheeler GS (1989) Compensatory increases in food consumption and utilization efficiencies by velvet bean caterpillars mitigate impact of diluted diets on growth. Entomol Exp Appl. 51:175–187
Stockoff BA (1993a) Ontogenetic change in dietary selection for protein and lipid by gypsy moth larvae. J Insect Physiol 39:677–686
Stockoff BA (1993b) Diet heterogeneity: implications for growth of a generalist herbivore, the gypsy moth. Ecology 74:1939–1949
Tebib K, Besancon P, Rouanet JM (1994) Dietary grape seed tannins affect lipoproteins, lipoprotein lipases, and lipids in rats fed hypercholesterolemic diet. Nutr J 12:2451–2457
Trakimas G, Krams R, Krama T, Kortet R, Haque S, Luoto S et al (2019) A link between developmental speed and physiological stress in an omnivorous insect. Front Behav Neurosci 13. https://doi.org/10.3389/fnbeh.2019.00042
Trier TM, Mattson WJ (2003) Diet-induced thermogenesis in insects: a developing concept in nutritional ecology. Environ Entomol 32:1–8
van der Meijden E, Wijn M, Verkaar HJ (1988) Defence and regrowth, alternative plant strategies in the struggle against herbivores. Oikos 51:355–363
Waldbauer G (1968) The consumption and utilization of food by insects. In: Advances in insect physiology, vol 5. Elsevier, pp 229-288
War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306–1320
Wetzel WC, Kharouba HM, Robinson M, Holyoak M, Karbon R (2016) Variability in plant nutrients reduces insect herbivore performance. Nature 539:425–427
White TCR (1993) The inadequate environment: nitrogen and the abundance of animals, 1st edn. Springer, Berlin
White TCR (2012) The inadequate environment: nitrogen and the abundance of animals, 2nd edn. Springer, Berlin
Zust T, Agrawal AA (2017) Trade-offs between plant growth and defense against insect herbivory: an emerging mechanistic synthesis. Annu Rev Plant Biol 68:513–534
Acknowledgements
We thank Christian Combs, John Christakis, Daytona Johnson, and Harry Price for technical assistance, Dr. Matthew Lehnert for the use of his lab and Percival, and the USDA-APHIS, Otis Air National Guard Base, Massachusetts, for providing egg masses. This research was supported by the Herrick Foundation, Kent State University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perkovich, C., Ward, D. Protein:Carbohydrate Ratios in the Diet of Gypsy Moth Lymantria dispar Affect its Ability to Tolerate Tannins. J Chem Ecol 46, 299–307 (2020). https://doi.org/10.1007/s10886-020-01161-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-020-01161-x