Abstract
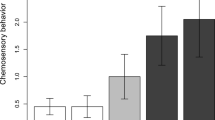
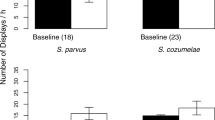
Animals rely on multimodal signals to obtain information from conspecifics through alternative sensory systems, and the evolutionary loss of a signal in one modality may lead to compensation through increased use of signals in an alternative modality. We investigated associations between chemical signaling and evolutionary loss of abdominal color patches in males of four species (two plain-bellied and two colorful-bellied) of Sceloporus lizards. We conducted field trials to compare behavioral responses of male lizards to swabs with femoral gland (FG) secretions from conspecific males and control swabs (clean paper). We also analyzed the volatile organic compound (VOC) composition of male FG secretions by stir bar extraction and gas chromatography-mass spectrometry (GC-MS) to test the hypothesis that loss of the visual signal is associated with elaboration of the chemical signal. Males of plain-bellied, but not colorful-bellied species exhibited different rates of visual displays when exposed to swabs of conspecific FG secretions relative to control swabs. The VOC composition of male Sceloporus FG secretions was similar across all four species, and no clear association between relative abundances of VOCs and evolutionary loss of abdominal color patches was observed. The emerging pattern is that behavioral responses to conspecific chemical signals are species- and context-specific in male Sceloporus, and compensatory changes in receivers, but not signalers may be involved in mediating increased responsiveness to chemical signals in males of plain-bellied species.




Similar content being viewed by others
References
Abell AJ (1998) The effect of exogenous testosterone on growth and secondary sexual character development in juveniles of Sceloporus virgatus. Herpetologica 54:533–543
Adkins-Regan E (2008) Do hormonal control systems produce evolutionary inertia? Philos Trans R Soc B 363:1599–1609
Alberts AC (1991) Phylogenetic and adaptive variation in lizard femoral gland secretions. Copeia 1991:69–79
Alberts AC, Sharp TR, Werner DI, Weldon PJ (1992) Seasonal variation of lipids in femoral gland secretions of male green iguanas Iguana iguana. J Chem Ecol 18:703–712
Angilleta MJ (2001) Thermal and physiological constraints on energy assimilation in a widespread lizard Sceloporus undulatus. Ecology 82:3044–3056
Apps PJ, Weldon PJ, Kramer M (2015) Chemical signals in terrestrial vertebrates: search for design features. Nat Prod Rep 32:1131–1153
Badyaev A, Hill G, Weckworth B (2002) Species divergence in sexually selected traits: Increase in song elaboration is related to decrease in plumage ornamentation in finches. Evolution 56:412–419
Baeckens S, Edwards S, Huyghe K, Van Damme R (2015) Chemical signaling in lizards: an interspecific comparison of femoral pore number in Lacertidae. Biol J Linn Soc 114:44–57
Berthou F, Picart D, Bardou L, Floch HH (1976) Separation of C19 and C21 dihyroxysteroids by open-hole tubular glass columns and lipophilic gel chromatography. J Chromatogr 118:135–155
Candolin U (2003) The use of multiple cues in mate choice. Biol Rev 78:575–595
Carpenter CC (1978) Comparative display behavior in the genus Sceloporus (Iguanidae). Cont Biol Geol Milwaukee Pub Mus 18:1–71
Carpenter CC, Ferguson GW (1977) Variation and evolution of stereotyped behaviour in reptiles. In Gans C, Tinkle DW (eds) Biology of the reptilia, Vol. 7 Ecology and behaviour A. Academic Press, New York, pp.335-554
Cooper WE, Greenburg N (1992) Reptilian coloration and behavior. In: Gans C, Crews D (eds) Hormones, brain, and behavior, biology of the reptilia, Vol. 18 Physiology E. University of Chicago Press, Illinois, pp. 298–422
Cox RM, Skelly SL, Leo A, John-Alder HB (2005) Testosterone regulates sexually dimorphic coloration in the eastern fence lizard, Sceloporus undulatus. Copeia 2005:597–608
Cox RM, Zilberman V, John-Alder HB (2008) Testosterone stimulates the expression of a social color signal in Yarrow’s Spiny Lizard, Sceloporus jarrovii. J Exp Zool Part A 309:505–514
Denoël M, Doellen J (2010) Displaying in the dark: light-dependent alternative mating tactics in the alpine newt. Behav Ecol Sociobiol 64:1171–1177
Duvall D (1979) Western fence lizard Sceloporus occidentalis chemical signals 1. Conspecific discriminations and release of a species typical visual display. J Exp Biol 210:321–326
Endler JA (1983) Natural selection and sexual selection on color patterns in poeciliid fishes. Environ Biol Fish 9:173–190
Escobar CA, Labra A, Niemeyer HM (2001) Chemical composition of precloacal secretions of Liolaemus lizards. J Chem Ecol 27:1677–1690
Font E, Barbosa D, Sampedro C, Carazo P (2012) Social behavior, chemical communication, and adult neurogenesis: studies of scent mark function in Podarcis wall lizards. Gen Comp Endocrinol 177:9–17
Fox SF, McCoy JK, Baird TA (2003) Lizard social behavior. The Johns Hopkins University Press, Baltimore
Gonzalez-Voyer A, Tex den RJ, Castelló A, Leonard JA (2013) Evolution of acoustic and visual signals in Asian barbets. J Evol Biol 26:647–659
Gordon SD, Uetz GW (2011) Multimodal communication of wolf spiders on different substrates: evidence for behavioural plasticity. Anim Behav 81:367–375
Hebets EA, Papaj DR (2005) Complex signal function: developing a framework of testable hypotheses. Behav Ecol Sociobiol 57:197–214
Hebets EA, Vink CJ, Sullivan-Beckers L, Rosenthal MF (2013) The dominance of seismic signaling and selection for signal complexity in Schizocosa multimodal courtship displays. Behav Ecol Sociobiol 67:1483–1498
Hews DK, Martins EP (2013) Visual and chemical signals of social communication: Providing the link to habitat and environment. In: Luttershcmidt WL (ed) Reptiles in research: Investigations of ecology, physiology, and behavior from desert to sea. Nova Science Publishers, New York, pp. 111–142
Hews DK, Date P, Hara E, Castellano MJ (2011) Field presentations of male secretions alter social display rates in Sceloporus virgatus but not in S. undulatus lizards. Behav Ecol Sociobiol 65:1403–1410
Kelso EC, Martins EP (2008) Effects of two courtship display components on female reproductive behaviour and physiology in the sagebrush lizard. Anim Behav 75:639–646
Ketterson ED, Atwell JW, McGlothlin JW (2009) Phenotype integration and independence: hormones, performance, and response to environmental change. Integr Comp Biol 49:365–379
López P, Martín J (2005a) Age-related differences in lipophilic compounds found in femoral gland secretions of male spiny-footed lizards, Acanthodactylus erythrurus. Z Naturforsch C 60:915–920
López P, Martín J (2005b) Chemical compounds from femoral gland secretions of male Iberian rock lizards, Lacerta monticola cyreni. Z Naturforsch C 60:632–636
Louw S, Burger B, Le Roux M, Van Wyk JH (2007) Lizard epidermal gland secretions: chemical characterization of the femoral gland secretions of the sungazer, Cordylus giganteus. J Chem Ecol 33:1806–1818
Manly BFJ (1991) Randomization, bootstrap and Monte Carlo methods in biology. Third edition. Chapman & Hall/CRC Press
Martín J, López P (2007) Scent may signal fighting ability in male Iberian rock lizards. Biol Lett 3:125–127
Martín J, López P (2015) Condition-dependent chemosignals in reproductive behavior of lizards. Horm Behav 68:14–24
Martín J, López P, Gabirot M, Pilz KM (2007) Effects of testosterone supplementation on chemical signals of male Iberian wall lizards: consequences for female mate choice. Behav Ecol Sociobiol 61:1275–1282
Martins EP, Ossip-Klein AG, Zúñiga-Vega JJ, Garcia CV, Campos SM, Hews DK (2015) Evolving from static to dynamic signals: evolutionary compensation between two communicative signals. Anim Behav 102:223–229
Mason RT, Parker MR (2010) Social behavior and pheromonal communication in reptiles. J Comp Physiol A 196:729–749
McGlothlin JW, Ketterson ED (2008) Hormone mediated suites as adaptations and evolutionary constraints. Philos Trans R Soc B 363:16110–11620
Moreira PL, López P, Martín J (2006) Femoral secretions and copulatory plugs convey chemical information about male identity and dominance status in Iberian rock lizards (Lacerta monticola). Behav Ecol Sociobiol 60:166–174
Muller L, Phillipou G (1987) Quantitation of 5α and 5β-androstanediols in urine by gas-chromatography mass spectrometry. Clin Chem 33:256–262
Ossip-Klein A, Fuentes JA, Hews DK, Martins EP (2013) Information content is more important than sensory system or physical distance in guiding the long-term evolutionary relationships between signaling modalities in Sceloporus lizards. Behav Ecol Sociobiol 67:1513–1522
Partan S (2004) Multisensory animal communication. In: Calvert G, Spence C, Stein BE (eds) The handbook of multisensory processes. MIT Press, Cambridge, pp. 225–240
Partan S, Marler P (1999) Communication goes multimodal. Science 283:1272–1273
Podos J (1997) A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes: Emberizidae). Evolution 51:537–551
Quinn VS, Hews DK (2010) The evolutionary decoupling of behavioral and color cues in a multicomponent signal in two Sceloporus lizards. Ethology 116:509–516
Shutler D, Weatherhead P (1990) Targets of sexual selection: Song and plumage of wood warblers. Evolution 44:1967–1977
Stuart-Fox DM, Moussalli A, Marshall NJ, Owens IPF (2003) Conspicuous males suffer higher predation risk: visual modeling and experimental evidence from lizards. Anim Behav 66:541–550
Swierk L, Ridgway M, Langkilde T (2012) Female lizards discriminate between potential reproductive partners using multiple male traits when territory cues are absent. Behav Ecol Sociobiol 66:1033–1043
Thompson JT, Bissell AN, Martins EP (2008) Inhibitory interactions between multimodal behavioral responses may influence the evolution of complex signals. Anim Behav 76:113–121
Weldon JP, Flachsbarth B, Schulz S (2008) Natural products from the integument of nonavian reptiles. Nat Prod Rep 25:738–756
Wiens JJ (1999) Phylogenetic evidence for multiple losses of a sexually selected character in phyrnosomatid lizards. Proc R Soc B 266:1529–1535
Wiens JJ (2001) Widespread loss of sexually selected traits: how the peacock lost its spots. Trends Ecol Evol 16:517–523
Wiens JJ, Kuczynski CA, Arif S, Reeder TW (2010) Phylogenetic relationships of phrynosomatid lizards based on nuclear and mitochondrial data, and a revised phylogeny for Sceloporus. Mol Pylogenet Evol 54:150–161
Acknowledgments
We thank Patrick Cain, Alison Ossip-Klein, and José Oyola Morales for assistance in the field. We thank Ryan Seddon, Savannah Price, and Lindsay Forrette for providing comments on a previous version of the manuscript. Permission to conduct this work was granted by the Texas Parks and Wildlife Department (S. merriami: SPR-0511-129) and the Secretaría de Medio Ambiente y Recursos Naturales of México (SEMARNAT; S. siniferus: 09/O1-0557/12/13; S. cozumelae and S. parvus: 09/k50904/01/13). We thank Big Bend Ranch State Park and El Parque Nacional Huatulco for granting access to public lands, and Carolyn Ohl-Johnson for granting access to private land. This material is based upon work supported by the National Science Foundation under grant numbers IOS-1050274 to EPM and IOS-1052247 to DKH, and while serving at the National Science Foundation (EPM). All procedures were approved by the Indiana State University Institutional Animal Care and Use Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pruett, J.A., Zúñiga-Vega, J.J., Campos, S.M. et al. Evolutionary Interactions Between Visual and Chemical Signals: Chemosignals Compensate for the Loss of a Visual Signal in Male Sceloporus Lizards. J Chem Ecol 42, 1164–1174 (2016). https://doi.org/10.1007/s10886-016-0778-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-016-0778-8