Abstract
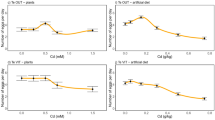
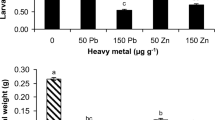
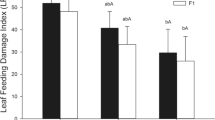
Metal hyperaccumulation may be an elemental defense, in which high concentrations of a metal in plant tissues decrease herbivore survival or growth rate. The Joint Effects Hypothesis suggests that a combination of metals, or a combination of a metal with an organic compound, may have an enhanced defensive effect. The enhancement may be additive or synergistic: in either case the concentration of a particular metal necessary to provide a defensive benefit for the plant is lowered. We tested the Joint Effects Hypothesis using Spodoptera exigua (beet armyworm) neonates fed artificial diets. Metal + metal experiments utilized diets amended with metal pairs, using four metals commonly hyperaccumulated by plants (Co, Cu, Ni, and Zn). We also conducted metal + organic compound experiments, pairing each metal with nicotine, mustard seed powder, or tannic acid. We tested for joint effects using both lethal (LC20 levels) and sublethal concentrations (10–25 % reduced larval weight) of the chemicals tested. For all experiments, either additive or synergistic effects were found. Of the metal + metal pairs tested, three (Co + Cu, Cu + Zn, and Ni + Zn) were synergistic in lethal concentration tests and only Co + Cu was synergistic in sublethal tests. For metal + organic combination lethal tests, synergism occurred for all combinations except for Co or Ni + nicotine, Ni + mustard seed powder, and Zn + nicotine. For sublethal tests, Zn + all three organic chemicals, Co + mustard seed powder or tannic acid, and Cu + nicotine, were synergistic. These results support the Joint Effects Hypothesis, suggesting that metals combined with other metals or organic compounds may be more effective against herbivores than individual metals.



Similar content being viewed by others
References
Abivardi C, Benz G (1984) Tests with the extracts of 21 medicinal plants for antifeedant activity against larvae of Pieris brassicae L. (Lep., Pieridae). Bull Soc Entomol Suisse 57:383–392
Ali MI, Luttrell RG (2009) Response estimates for assessing Heliothine susceptibility to Bt toxins. J Econ Entomol 102:1935–1947
Anilkumar KJ, Sivasupramaniam S, Head G, Orth R, Van Santen E, Moar WJ (2009) Synergistic interactions between Cry1Ac and natural cotton defenses limit survival of Cry1Ac-resistant Helicoverpa zea (Lepidoptera: Noctuidae) on Bt cotton. J Chem Ecol 35:785–795
Bennett RN, Wallsgrove RM (1994) Secondary metabolites in plant defense mechanisms. New Phytol 127:617–633
Boyd RS (2014) Ecology and evolution of metal-hyperaccumulating plants. In: Rajakaruna N, Boyd RS, Harris TB (eds) Plant ecology and evolution in harsh environments. Nova, Happauge, pp 227–241
Boyd RS (2012) Plant defense using toxic inorganic ions: conceptual models of the defensive enhancement and joint effects hypotheses. Plant Sci 195:88–95
Boyd RS (2007) The defense hypothesis of elemental hyperaccumulation: status, challenges and new directions. Plant Soil 293:153–176
Boyd RS, Martens SN (1992) The raison d’etre for metal hyperaccumulation by plants. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils. Intercept Limited, Andover, pp 279–289
Boyd RS, Moar WJ (1999) The defensive function of Ni in plants: response of the polyphagous herbivore Spodoptera exigua (Lepidoptera: Noctuidae) to hyperaccumulator and accumulator species of Streptanthus (Brassicaceae). Oecologia 118:218–224
Capinera JL (2006) Beet Armyworm, Spodoptera exigua (Hübner)(Insecta: Lepidoptera: Noctuidae). Entomology and Nematology Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida
Cheruiyot DJ, Boyd RS, Moar WJ (2013) Exploring lower limits of plant elemental defense by cobalt, copper, nickel and zinc. J Chem Ecol 39:666–674
Clancy KM, Price PW (1987) Rapid herbivore growth enhances enemy attack: sublethal plant defenses remain a paradox. Ecology 68:733–737
Coleman CM, Boyd RS, Eubanks MD (2005) Extending the elemental defense hypothesis: dietary metal concentrations below hyperaccumulator levels could harm herbivores. J Chem Ecol 31:1669–1681
Davis MA, Boyd RS (2000) Dynamics of Ni-based defense and organic defenses in the Ni hyperaccumulator, Streptanthus polygaloides (Brassicaceae). New Phytol 146:11–217
Davis MA, Pritchard SG, Boyd RS, Prior SA (2001) Developmental and induced responses of nickel-based and organic defenses of nickel-hyperaccumulating shrub, Psychotria douarrei. New Phytol 150:49–58
Dyer LA (2011) New synthesis—Back to the future: new approaches and directions in chemical studies of coevolution. J Chem Ecol 37:669
Gershenzon J, Fontana A, Burow M, Wittstock U, Degenhardt J (2012) Mixtures of plant secondary metabolites: metabolic origins and ecological benefits. In: Iason GR, Dicke M, Hartley SE (eds) The ecology of plant secondary metabolites: from genes to global processes. Cambridge University Press, New York, pp 56–77
Greenberg SM, Sappington TW, Legaspi BC, Liu T-X, Sétamou M (2001) Feeding and life history of Spodoptera exigua (Lepidoptera: Noctuidae) on different host plants. Ann Entomol Soc Am 94:566–575
Hodgson E, Das PC, Cho TM, Rose RL (2008) Chapter 10. Phase 1 metabolism of toxicants and metabolic interactions. In: Stuart RC, Hodgson E (eds) Molecular and biochemical toxicology, 4th edn. John Wiley and Sons, Hoboken, pp 173–203
Hörger AC, Fones HN, Preston GM (2013) The current status of the elemental defense hypothesis in relation to pathogens. Front Plant Sci. doi:10.3389/fpls.2013.00395
Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59:41–66
Jaffré T, Brooks RR, Lee J, Reeves RD (1976) Sebertia acuminata: a hyperaccumulator of nickel from New Caledonia. Science 193:579–580
Jensen PD, Johnson LR, Trumble JT (2006) Individual and joint actions of selenate and methylmercury on the development and survival of insect detritivore Megaselia scalaris. Arch Environ Contam Toxicol 50:523–530
Jensen PD, Sorensen MA, Walton WE, Trumble JT (2007) Lethal and sublethal responses of an aquatic insect Culex quinquefasciatus (Diptera: Culicidae) challenged with individual and joint exposure to dissolved sodium selenite and methylmercury chloride. Environ Toxicol 22:287–294
Jhee EM, Boyd RS, Eubanks MD (2006) Effectiveness of metal-metal and metal-organic compound combinations against Plutella xylostella (Lepidoptera: Plutellidae): implications for plant elemental defense. J Chem Ecol 32:239–259
Kazemi-Dinan A, Thomaschky S, Stein RJ, Krämer U, Müller C (2014) Zinc and cadmium hyperaccumulation act as deterrents towards specialist herbivores and impede the performance of a generalist herbivore. New Phytol. doi:10.1111/nph.12663
Li G, Pang Y, Chen Q, Su Z, Wen X (2002) Studies on the artificial diet for Beet Armyworm, Spodoptera exigua. Chin J Biol Control 18:132–134
Martens SN, Boyd RS (1994) The ecological significance of nickel hyperaccumulation: a plant chemical defense. Oecologia 98:379–384
Mazid M, Khan TA, Mohammad F (2011) Role of secondary metabolites in defense mechanisms of plants. Biol Med 3(2):232–249
Mithöfer Z, Boland W (2012) Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63:431–450
Morrison RS, Brooks RR, Reeves RD, Malaisse F (1979) Copper and cobalt uptake by metallophytes from Zaïre. Plant Soil 53:535–539
Moyes CL, Collin HA, Britton G, Raybould AF (2000) Glucosinolates and differential herbivory in wild populations of Brassica oleracea. J Chem Ecol 26:2625–2641
Nelson AC, Kursar TA (1999) Interactions among plant defense compounds: a method for analysis. Chemoecology 2:81–92
Nomura M, Itioka T (2002) Effects of synthesized tannin on the growth and survival of a generalist herbivorous insect, the common cutworm, Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Appl Entomol Zool 37:285–289
Price PW, Bouton CE, Gross P, McPherson BA, Thompson JN, Weis AE (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Evol Syst 11:41–65
Rasmann S, Agrawal AA (2009) Plant defense against herbivory: progress in identifying synergism, redundancy, and antagonism between resistance traits. Curr Opin Plant Biol 12:473–478
Reeves RD, Baker AJM (2000) Metal-accumulating plants. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: using plants to clean up the environment. John Wiley and Sons, New York, pp 193–229
Reeves RD, Baker AJM (1984) Studies on metal uptake by plants from serpentine and non-serpentine populations of Thlaspi goesingense Halacsy (Cruciferae). New Phytol 98:191–204
Robertson JL, Preisler HK, Russell RM (2007) PoloPlus: Probit and Logit Analysis. LeOra Software, Berkeley
Salama HS, Foda MS, Zaki FN, Moawad S (1984) Potency of combinations of Bacillus thuringiensis and chemical insecticides on Spodoptera littoralis (Lepidoptera: Noctuidae). J Econ Entomol 77:885–890
Sambamurty AVSS (2006) A textbook of plant pathology. I.K. International Pvt. Ltd., New Delhi
SAS Institute (2005) StatView 5.0. Thomson—Brooks/Cole, Belmont
Tang Y-T, Qiu R-L, Zeng X-W, Ying R-R, Yu F-M, Zhou X-Y (2009) Lead, zinc, cadmium hyperaccumulation and growth stimulation in Arabis paniculata Franch. Environ Exp Bot 66:126–134
Thamthiankul S, Moar WJ, Miller ME, Panbangred W (2004) Improving the insecticidal activity of Bacillus thuringiensis subsp. aizawai against Spodoptera exigua by chromosomal expression of a chitinase gene. Appl Microbiol Biotechnol 65:183–192
Tolrá RS, Poschenrieder C, Alonso R, Barcélo D, Barcélo J (2001) Influence of zinc hyperaccumulation on glucosinolates in Thlaspi caerulescens. New Phytol 151:621–626
Trumble JT, Kund GS, White KK (1998) Influence of form and quantity of selenium on the development and survival of an insect herbivore. Environ Pollut 101:175–182
Van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334
Wink M (2008) Plant secondary metabolism: diversity, function and its evolution. Nat Prod Commun 3:1205–1216
Wise MJ, Fox RJ, Abrahamson WG (2006) Disarming the paradox of sublethal plant defense against insects: Trirhabda virgata larval development time and leaf tissue loss on Solidago altissima. Entomol Exp Appl 120:77–87
Acknowledgments
This research was supported by Alabama Agricultural Experiment Station Project No. ALA021-1-09008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheruiyot, D.J., Boyd, R.S. & Moar, W. Testing the Joint Effects Hypothesis of Elemental Defense using Spodoptera Exigua . J Chem Ecol 41, 168–177 (2015). https://doi.org/10.1007/s10886-015-0553-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-015-0553-2