Abstract
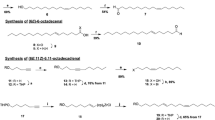
The promethea moth Callosamia promethea is one of three species of silkmoths from the genus Callosamia that occur in North America. Cross attraction of males to heterospecific calling females has been observed in the field, and hybrid progeny have been produced by pairing heterospecifics in captivity. These observations suggest that all three species share or have considerable overlap in the sex attractant pheromones produced by females, so that other prezygotic isolating mechanisms, such as diel differences in reproductive activity, limit hybridization in the field. Coupled gas chromatography-electroantennogram detection and gas chromatography- mass-spectrometry analyses of extracts of volatiles collected from female promethea moths supported the identification of (4E,6E,11Z,13Z)-hexadeca-4,6,11,13-tetraenal [(4E,6E,11Z,13Z)-16:Ald] as the compound in extracts that elicited the largest responses from antennae of males. The identification was confirmed by non-selective synthesis of several isomers as analytical standards, and stereoselective synthesis of (4E,6E,11Z,13Z)-16:Ald for testing in field trials. Male moths were strongly attracted to synthetic (4E,6E,11Z,13Z)-16:Ald, suggesting that this compound is the major and possibly the only component of the sex pheromone of these large saturniid moths. Based on the cross-attraction of heterospecifics, it is likely that this is also a major pheromone component of the other two North American Callosamia species as well.





Similar content being viewed by others
References
Bayer A, Maier ME (2004) Synthesis of enamides from aldehydes and amides. Tetrahedron 60:6665–6677
Bestmann HJ, Attygalle AB, Schwarz J, Garbe W, Vostrowsky O, Tomida I (1989) Pheromones. 71. Identification and synthesis of female sex pheromone of eri-silkworm, Samia cynthia ricini (Lepidoptera: Saturniidae). Tetrahedron Lett 30:2911–2914
Boettner GH, Elkinton JS, Boettner CJ (2000) Effects of a biological control introduction on three nontarget native species of saturniid moths. Conserv Biol 14:1798–1806
Brown LN (1972) Mating behavior and life habits of the sweet-bay silk moth (Callosamia carolina). Science 176:73–75
Brown HC, Mandal AK, Kulkarni SU (1977) Hydroboration. 45. New, convenient preparations of representative borane reagents utilizing borane-methyl sulfide. J Org Chem 42:1392–1398
Collins MM, Weast RD (1961) Wild silk moths of the United States. Collins Radio Co., Cedar Rapids
El-Sayed A (2012) The Pherobase: Database of pheromones and semiochemicals. http://www.pherobase.com
Ferguson DC (1972) Fasc. 20.2B, Bombicoidea (in part). In: Dominick RB et al (eds) The moths of North America north of Mexico. Classey, London, pp 243–275
Haskins CP, Haskins EF (1958) Note on the inheritance of behavior patterns for food selection and cocoon spinning in F1 hybrids of Callosamia promethea × C. angulifera. Behavior 13:89–95
Holden C (1992) Thousands of insects “enroll” at Yale. Science 256:313
Johnson KS, Scriber JM, Nitao JK, Smitley DR (1995) Toxicity of Bacillus thuringiensis var kurstaki to three nontarget Lepidoptera in field studies. Environ Entomol 24:288–297
Jurberg ID, Odabchian Y, Gagosz Y (2010) Hydroalkylation of alkynyl ethers via a gold(I)-catalyzed 1,5-hydride shift/cyclization sequence. J Am Chem Soc 132:3543–3552
Kochansky J, Tette J, Taschenberg EF, Cardé RT, Kaissling K-E, Roelofs WL (1975) Sex pheromone of the moth Antheraea polyphemus. J Insect Physiol 21:1977–1983
Millar JG, McElfresh JS, Romero C, Vila M, Mari-Mena N, López-Vaamonde C (2010) Identification of the sex pheromone of a protected species, the Spanish moon moth Graellsia isabellae. J Chem Ecol 36:923–932
Mukai C, Sugimoto Y-I, Hanoaka M (1998) A new procedure for highly stereoselective and regioselective synthesis of 2-ethynyl-3-hydroxytetrahydropyran derivatives based on alkyne-Co2(CO)6 complex. Tetrahedron 54:823–850
Organ MG, Ghasemi H (2004) Metal-catalyzed coupling reactions on an olefin template: the total synthesis of (13E,15E,18Z,20Z)-1-hydroxypentacosa-13,15,18,20-tetraen-11-yn-4-one 1-acetate. J Org Chem 69:695–700
Peigler RS (1977) Hybridization of Callosamia (Saturniidae). J Lepidopterol Soc 31:23–34
Peigler RS (1980) Demonstration of reproductive isolating mechanisms in Callosamia (Saturniidae) by artificial hybridization. J Res Lepidoptera 19:72–81
Rau P, Rau N (1929) The sex attraction and rhythmic periodicity in giant saturniid moths. Trans Acad Sci St Louis 26:81–221
Remington CL (1968) Suture-zones of hybrid interaction between recently joined biota. Evol Biol 2:321–428
Ribe S, Kondru RK, Beratan DN, Wipf P (2000) Optical rotation computation, total synthesis, and stereochemistry assignment of the marine natural product pitiamide A. J Am Chem Soc 122:4608–4617
Schweitzer DF (1988) Status of Saturniidae in the northeastern USA: a quick review. News Lepidopterol Soc 1:4–5
Skinner H (1914) Callosamia promethea and angulifera (Lepid.). Entomol News 25:468–469
Sonnet PE, Heath RR (1980) Stereospecific synthesis of (Z, Z)-11,13-hexadecadienal, a female sex pheromone of the navel orangeworm, Amyelois transitella (Lepidoptera: Pyralidae). J Chem Ecol 6:221–228
Sparling BA, Simpson GL, Jamison TF (2009) Strategic use of nickel(0)-catalyzed enyne-epoxide reductive coupling toward the synthesis of (−)-cyatha-3,12-diene. Tetrahedron 65:3270–3280
Toliver ME, Sternburg JG, Waldbauer GP (1979) Daily flight periods of male Callosamia promethea (Saturniidae). J Lepidopterol Soc 33:232–238
Tuskes PM, Tuttle JP, Collins MM (1996) The wild silk moths of North America: a natural history of the Saturniidae of The United States and Canada. Cornell University Press, Ithaca
Waldbauer GP, Sternburg JG (1985) Adult emergence in two univoltine Callosamia promethea populations: preponderance of the early emerging morph in the north and the late emerging morph in the south (Lepidoptera: Saturniidae). Great Lakes Entomol 18:139–142
Acknowledgments
We are indebted to the Ministry of Science and Innovation for a FPI fellowship to RG. We also thank Shelby Stamper for assistance with the field trials at the UK Arboretum and North Farm. Financial assistance was provided to JDA by the LSU AgCenter, to KFH by Kentucky Agricultural Experiment Station, to AG by CICYT through project AGL2009-13452-C02-01, and to JGM through Hatch project CA-R*-5181-H.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supporting information includes descriptions of the syntheses of isomeric mixtures of (4E,6E,11Z/E,13E)-hexadeca-4,6,11,13-tetraenal (20a), (4E,6E,11E/Z,13Z)-hexadeca-4,6,11,13-tetraenal (20b) and (4E,6E,11E,13Z/E)- hexadeca-4,6,11,13-tetraen-1-ol (23), and a video showing the responses of male moths to pheromone lures.
ESM 1
(DOC 336 kb)
Close range response of the Promethea moth, Callosamia promethea, to 100 micrograms of E4,E6,Z11,Z13-16:Ald on a red rubber septum, May 2013, Lexington, Kentucky, USA. (M4V 6.46 mb)
Rights and permissions
About this article
Cite this article
Gago, R., Allison, J.D., McElfresh, J.S. et al. A Tetraene Aldehyde as the Major Sex Pheromone Component of the Promethea Moth (Callosamia promethea (Drury)). J Chem Ecol 39, 1263–1272 (2013). https://doi.org/10.1007/s10886-013-0349-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-013-0349-1