Abstract
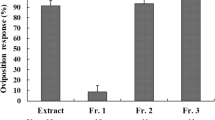
Papilio maackii females prefer a rutaceous plant, Phellodendron amurense, for oviposition, whereas another semi-sympatric Rutaceae feeder, Papilio protenor, never exploits this plant as a host in nature. However, the larvae of both species perform well on this plant in the laboratory. Phellamurin, a flavonoid present in the organic fraction from P. amurense inhibits egg laying by P. protenor. We examined whether phellamurin is involved in the differential acceptance of P. amurense by the two butterflies. The ovipositing females of P. maackii readily accepted P. amurense and a methanolic extract of the foliage, while P. protenor rejected them entirely. However, the aqueous fraction derived from the extract elicited significant oviposition responses of similar levels from the two species. Phellamurin did not induce oviposition behavior in P. protenor females. In contrast, P. maackii was stimulated to oviposit by phellamurin at concentrations exceeding 0.2%. The response was dose-dependent and reached ca. 70% at 2% phellamurin, which is approximately equivalent to its natural abundance in young leaves of P. amurense. Since the aqueous fraction was very stimulatory to both species, the combined effect of phellamurin and the aqueous fraction on oviposition was tested. The addition of phellamurin to the aqueous fraction enhanced the ovipositional activity of P. maackii, but dramatically suppressed the oviposition response of P. protenor even at 0.1% concentration. These results, taken together with those obtained from electrophysiological recordings with foretarsal chemosensilla, indicate that phellamurin acts as an oviposition stimulant for P. maackii, and as a potent deterrent for P. protenor. The results suggest that host range expansion or host shifts may be made by ovipositing females that overcome phytochemical barriers.




Similar content being viewed by others
References
Abad-García, B., Garmón-Lobato, S., Berrueta, L. A., Gallo, B., and Vicente, F. 2009. A fragmentation study of dihydroquercetin using triple quadrupole mass spectrometry and its application for identification of dihydroflavonols in Citrus juices. Rapid Commun. Mass Spectrom. 23:2785–2792.
Aubert, J., Legal, L., Descimon, H., and Michel, F. 1999. Molecular phylogeny of swallowtail butterflies of the tribe Papilionini (Papilionidae, Lepidoptera). Mol. Phylogenet. Evol. 12:156–167.
Berenbaum, M. R. 1991. Comparative processing of allelochemicals in the Papilionidae (Lepidoptera). Arch. Insect Biochem. Physiol. 17:213–221.
Bernays, E. A. 2001. Neural limitations in phytophagous insects: Implications for diet breadth and evolution of host affiliation. Annu. Rev. Entomol. 46:703–727.
Chachin, M., Honda, K., and Ômura, H. 2007. Appraisal of the acceptability of subtropical rutaceous plants for a swallowtail butterfly, Papilio protenor demetrius (Lepidoptera: Papilionidae). Appl. Entomol. Zool. 42:121–128.
Du, Y.-J., Van Loon, J. J. A., and Renwick, J. A. A. 1995. Contact chemoreception of oviposition-stimulating glucosinolates and an oviposition-deterrent cardenolide in two subspecies of Pieris napi. Physiol. Entomol. 20: 164–174.
Endo, S., and Nihira, I. 1990. Larval Food of Japanese Butterflies. Group Tamamushi, Tokyo (in Japanese).
Feeny, P. 1991. Chemical constraints on the evolution of swallowtail butterflies, pp. 315–340, in P. W. Price, T. M. Lewinsohn, G. W. Fernandes, W. W. and Benson (eds.). Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. Wiley & Sons, New York.
Harborne, J. B., and Baxter, H. 1993. Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants. Taylor & Francis Ltd., London.
Honda, K. 1990. Identification of host-plant chemicals stimulating oviposition by swallowtail butterfly, Papilio protenor. J. Chem. Ecol. 16:325–337
Honda, K. 1995. Chemical basis of differential oviposition by lepidopterous insects. Arch. Insect Biochem. Physiol. 30:1–23.
Honda, K. 2005. Larval feeding habit and host selection, pp. 255–301, in K. Honda, Y. Kato (eds.). Biology of Butterflies. University of Tokyo Press, Tokyo (in Japanese).
Honda, K., and Hayashi, N. 1995a. Chemical factors in rutaceous plants regulating host selection by two swallowtail butterflies, Papilio protenor and P. xuthus (Lepidoptera:Papilionidae). Appl. Entomol. Zool. 30:327–334.
Honda, K., and Hayashi, N. 1995b. A flavonoid glucoside, phellamurin, regulates differential oviposition on a rutaceous plant, Phellodendron amurense, by two sympatric swallowtail butterflies, Papilio protenor and P. xuthus: The front line of a coevolutionary arms race? J. Chem. Ecol. 21:1531–1539.
Honda, K., Ômura, H., Hori, M., and Kainoh, Y. 2010. Allelochemicals in plant-insect interactions, pp. 563–594, in L. Mander and H.-W. Lui (eds.). Comprehensive Natural Products II. Chemistry and Biology. Elsevier, Oxford.
Inoue, T. A. 2006. Morphology of foretarsal ventral surfaces of Japanese Papilio butterflies and relations between these morphology, phylogeny and hostplant preferring hierarchy. Zool. Sci. 23:169–189.
Mercader, R. J., and Scriber, J. M. 2008. Divergence in the ovipositional behavior of the Papilio glaucus group. Insect Sci. 15:361–367.
Murakami, T., Honda, K., Nakayama, T., and Hayashi, N. 2003. Phytochemical-mediated differential acceptance of four rutaceous plants by a swallowtail butterfly, Papilio polytes (Lepidoptera: Papilionidae). Appl. Entomol. Zool. 38:37–43.
Nakayama, T., Honda, K., and Hayashi, N. 2002. Chemical mediation of differential oviposition and larval survival on rutaceous plants in a swallowtail butterfly, Papilio polytes. Entomol. Exp. Appl. 105:35–42.
Nakayama, T., Honda, K., Ômura, H., and Hayashi, N. 2003. Oviposition stimulants for the tropical swallowtail butterfly, Papilio polytes, feeding on a rutaceous plant, Toddalia asiatica. J. Chem. Ecol. 29:1621–1634.
Nishida, R., Ohsugi, T., Kokubo, S., and Fukami, H. 1987. Oviposition stimulants of a Citrus-feeding swallowtail butterfly, Papilio xuthus L. Experientia 43: 342–344.
Nishida, R., Ohsugi, T., Fukami, H., and Nakajima, S. 1990. Oviposition deterrent of a Rutaceae-feeding swallowtail butterfly, Papilio xuthus, from a non-host rutaceous plant, Orixa japonica. Agric. Biol. Chem. 54:1265–1270.
Ohsugi, T., Nishida, R., and Fukami, H. 1985. Oviposition stimulants of Papilio xuthus, a Citrus-feeding swallowtail butterfly. Agric. Biol. Chem. 49:1897–1900.
Qiu, Y.-T., Van Loon, J. J. A., and Roessingh, P. 1998. Chemoreception of oviposition inhibiting terpenoids in the diamondback moth Plutella xylostella. Entomol. Exp. Appl. 87:143–155.
Renwick, J. A. A., and Chew, F. S. 1994. Oviposition behavior in Lepidoptera. Annu. Rev. Entomol. 39:377–400.
Ribeiro, A. B., Abdelnur, P. V., Garcia, C. F., Belini, A., Severino, V. G. P., Silva, M. F. das G. F. da, Fernandes, J. B., Vieira, P. C., De Carvalho, S. A., De Souza, A. A., and Machado, M. A. 2008. Chemical characterization of Citrus sinensis grafted on C. limonia and the effect of some isolated compounds on the growth of Xylella fastidiosa. J. Agric. Food Chem. 56:7815–7822.
Roessingh, P., Städler, E., Shöni, R., and Feeny, P. 1991. Tarsal contact chemoreceptors of the black swallowtail butterfly Papilio polyxenes: responses to phytochemicals from host- and non-host plants. Physiol. Entomol. 16: 485–495.
Ryan, M. F. 2002. Insect Chemoreception Fundamental and Applied. Kluwer Academic Publishers, Dordrecht.
Schoonhoven, L. M., and Fu-Shun, Y. 1989. Interference with normal chemoreceptor activity by some sesquiterpenoid antifeedants in an herbivorous insect Pieris brassicae. J. Insect Physiol. 35:725–728.
Schoonhoven, L. M., Van Loon, J.A.A., and Dicke, M. 2005. Insect-Plant Biology, 2nd Edition. Oxford University Press, Oxford, UK.
Scriber, J. M. 1995. Overview of swallowtail butterflies: Taxonomic and distributional latitude, pp. 3–8, in J. M. Scriber, Y. Tsubaki and R. C. Lederhouse (eds.). Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scientific Publ., Gainesville.
Scriber, J. M., Larsen, M. L., and Zalucki, M. P. 2007. Papilio aegeus Donovan (Lepidoptera: Papilionidae) host plant range evaluated experimentally on ancient angiosperms. Aust. J. Entomol. 46:65–74.
Scriber, J. M., Larsen, M. L., Allen, G. R., Walker, P. W., and Zalucki, M. P. 2008. Interactions between Papilionidae and ancient Australian angiosperms: evolutionary specialization or ecological monophagy? Entomol. Exp. Appl. 128:230–239.
Städler, E., Renwick, J. A. A., Radke, C. D., and Sachdev-Gupta, K. 1995. Tarsal contact chemoreceptor responses to glucosinolates and cardenolides mediating oviposition in Pieris rapae. Physiol. Entomol. 20:175–187.
Thompson, J. N., Wehling, W., and Podolsky, R. 1990. Evolutionary genetics of host use in swallowtail butterflies. Nature, 344: 148–150.
Tripoli, E., Guardia, M. L., Giammanco, S., Majo, D. D., and Giammanco, M. 2007. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 104:466–479.
Van Loon, J. J. A. 1996. Chemosensory basis of feeding and oviposition behaviour in herbivorous insects: a glance at the periphery. Entomol. Exp. Appl. 80:7–13.
Yagi, T., Sasaki, G.., and Takebe, H. 1999. Phylogeny of Japanese papilionid butterflies inferred from nucleotide sequences of the mitochondrial ND5 gene. J. Mol. Evol. 48:42–48.
Zakharov, E. V., Caterino, M. S., and Sperling, F. A. 2004. Molecular phylogeny, historical biogeography, and divergence time estimates for swallowtail butterflies of the genus Papilio (Lepidoptera: Papilionidae). Syst. Biol. 53:193–215.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to K. Honda (No. 14560039).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Honda, K., Ômura, H., Chachin, M. et al. Synergistic or Antagonistic Modulation of Oviposition Response of Two Swallowtail Butterflies, Papilio maackii and P. protenor, to Phellodendron amurense by Its Constitutive Prenylated Flavonoid, Phellamurin. J Chem Ecol 37, 575–581 (2011). https://doi.org/10.1007/s10886-011-9965-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-011-9965-9