Abstract
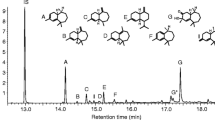
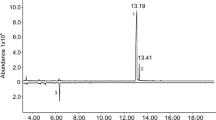
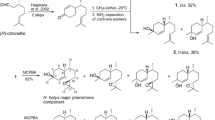
The male-produced aggregation pheromones of Tetropium fuscum (F.) and T. cinnamopterum Kirby were identified as (2S,5E)-6,10-dimethyl-5,9-undecadienol by chemical analysis, synthesis, electronantennography, and field trapping; the compound is here renamed “fuscumol”. The effect of fuscumol chirality, alone or with host volatiles, and fuscumol release rate on Tetropium spp. was tested in field-trapping experiments in Nova Scotia and Poland. Both (S)-fuscumol and racemic fuscumol synergized trap catches of male and female T. fuscum, T. cinnamopterum, and T. castaneum (L.) when combined with a blend of host monoterpenes and ethanol. Without added host volatiles, fuscumol was either unattractive (in Nova Scotia) or only slightly so (in Poland). (R)-Fuscumol, alone or in combination with host volatiles, did not elicit increases in trap capture of any Tetropium species, relative to the controls. Fuscumol synergized attraction of both sexes to host volatiles, thus indicating it acts as an aggregation pheromone. Sex ratio was often female-biased in traps baited with fuscumol plus host volatiles, and was either unbiased or male-biased in traps with host volatiles alone. In traps with host volatiles and racemic fuscumol, mean catches of Tetropium species were unaffected by fuscumol release rates ranging from 1 to 32 mg/d. The attraction of three different Tetropium species to the combination of (S)-fuscumol and host volatiles suggests that cross-attraction may occur where these species are sympatric, and that reproductive isolation possibly occurs via differences in close-range cues. These results have practical applications for survey and monitoring of T. fuscum, a European species established in Nova Scotia since at least 1980, and for early detection of T. castaneum, a European species not presently established in North America.






Similar content being viewed by others
References
Allison, J. D., Borden, J. H., and Seybold, S. J. 2004. A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 14:123–150.
Borden, J. H. 1974. Aggregation pheromones in the Scolytidae. pp. 135–60 in M. C. Birch (ed.). Pheromones. North Holland Pub. Co, Amsterdam.
Cunningham, G. 2010. Update on the Brown Spruce Longhorn Beetle (BSLB), Tetropium fuscum (Fabricius), in Nova Scotia, Canada. North American Plant Protection Organization Phytosanitary Alert System Official Pest Report. http://www.pestalert.org/oprDetail.cfm?oprID=413 (accessed 23 April 2010).
Fonseca, M. G., Vidal, D. M., and Zarbin, P. H. G. 2010. Male-produced sex pheromone of the Cerambycid beetle Hedypathes betulinus: Chemical identification and biological activity. J. Chem. Ecol. doi:10.1007/s10886-010-9850-y
Furniss, R. L., and Carolin, V. M. 1980. Western Forest Insects. U.S. Dept. Agriculture Forest Service Miscellaneous Publication No. 1339, 654 p.
Ginzel, M. D., and Hanks, L. M. 2003. Contact pheromones as mate recognition cues of four species of longhorned beetles (Coleoptera: Cerambycidae). J. Insect Behav. 16:181–187.
Gries, R., Khaskin, G., Daroogheh, H., Mart, C., Karadag, S., Kubilayer, M., Britton, R., and Gries, G. 2006. (2S,12Z)-2-Acetoxy-12-heptadecene: major sex pheromone component of pistachio twig borer, Kermania pistaciella. J. Chem. Ecol. 32:2667–2677.
Hanks, L. M., Millar, J. G., Moreira, J. A., Barbour, J. D., Lacey, E. S., Mc Elfresh, J. S., Reuter, F. R., and Ray, A. M. 2007. Using generic pheromone lures to expedite identification of aggregation pheromones for the cerambycid beetles Xylotrechus nauticus, Phymatodes lecontii, and Neoclytus modestus modestus. J. Chem. Ecol. 33:889–907.
Hick, A. J., Luszniak, M. C., and Pickett, J. A. 1999. Volatile isoprenoids that control insect behavior and development. Nat. Prod. Rep. 16:39–54.
Jacobs, K., Seifert, K. A., Harrison, K. J., and Kirisits, T. 2003. Identity and phylogenetic relationships of ophiostomatoid fungi associated with invasive and native Tetropium species (Coleoptera: Cerambycidae) in Atlantic Canada. Can J. Bot. 81:316–329.
Juutinen, P. 1955. Zur Biologie und forstlichen Bedeutung der Fichtenböcke (Tetropium Kirby) in Finland. Acta Entomol. Fenn. 11:1–112.
Kosugi, H., Yamabe, O., and Kato, M. 1998. Synthetic study of marine lobane diterpenes: efficient synthesis of (1)-fuscol. J. Chem. Soc., Perkin Trans. 1:217–221.
Lacey, E. S., Ginzel, M. D., Millar, J. G., and Hanks, L. M. 2004. Male-produced aggregation pheromone of the cerambycid beetle Neoclytus acuminatus acuminatus. J. Chem. Ecol. 30:1493–1507.
Lacey, E. S., Moreira, J. A., Millar, J. G., Ray, A. M., and Hanks, L. M. 2007. Male-produced aggregation pheromone of the cerambycid beetle Neoclytus mucronatus mucronatus. Entomol. Exp. Appl. 122:171–179.
Lacey, E. S., Moreira, J. A., Millar, J. G., and Hanks, L. M. 2008. A male-produced aggregation pheromone blend consisting of alkanediols, terpenoids, and an aromatic alcohol from the Cerambycid beetle Megacyllene caryae. J. Chem. Ecol. 34:408–417.
Landolt, P. J., and Phillips, T. W. 1997. Host plant influences on sex pheromone behavior of phytophagous insects. Annu. Rev. Entomol. 42:371–91.
Lemay, M. A., Silk, P. J., and Sweeney, J. D. 2010. Calling behaviour of Tetropium fuscum (Coleoptera: Cerambycidae: Spondylidinae). Can. Entomol. 141:256-260.
Linsley, E. G. 1961. The Cerambycidae of North America, Part I. Introduction. Univ. Calif. Publ. Entomol. 18:1–135.
Madyastha, K. M., and Gururaja, T. L. 1994. Asymmetric reduction of prochiral ketones by cell-free systems from Alcaligenes eutrophus. J. Chem. Tech. Biotechnol. 59:249–255.
Miller, D. R., Borden, J. H., and Lindgren, B. S. 2005. Dose-dependent pheromone responses of Ips pini, Orthotomicus latidens (Coleoptera: Scolytidae), and associates in stands of lodgepole pine. Environ. Entomol. 34:591–597.
Mori, K. 1975. Synthesis of optically active forms of sulcatol. Tetrahedron 31:3011–3012.
Mori, K. 2007. Significance of chirality in pheromone science. Bioorg. Medic. Chem. 15:7505–7523.
Nehme, M.E., Keena, M. A., Zhang, A., Baker, T. C., and Hoover, K. 2009. Attraction of Anoplophora glabripennis to male-produced pheromone and plant volatiles. Environ. Entomol. 38:1745–1755
Oritami, T. and Yamashita, K. 1973. Microbial resolution of (±)-acyclic alcohols. Agr. Biol. Chem. 37:1923–1928.
Pajares, J. A., Álvarez, G., Ibeas, F., Gallego, D., Hall, D. R., and Farman, D. I. 2010. Identification and field activity of a male-produced aggregation pheromone in the pine sawyer beetle, Monochamus galloprovincialis. J. Chem. Ecol. published online 02 May 2010. doi:10.1007/s10886-010-9791-5.
Raffa, K. F., Phillips, T. W., and Salom, S. M. 1993. Strategies and mechanisms of host colonization by bark beetles, pp. 103–128, in T. D. Schowalter and G. M. Filip (eds.). Beetle-Pathogen Interactions in Conifer Forests. Academic Press, London.
Ray, A. M., Millar, J. G., Mcelfresh, J. S., Swift, I. P., Barbour, J.D., and Hanks, L. M. 2009. Male-produced aggregation pheromone of the cerambycid beetle Rosalia funebris. J. Chem. Ecol. 35:96–103.
Reddy, G. V. P., Fettköther, R., Noldt, U., and Dettner, K. 2005a. Capture of female Hylotrupes bajulus as influenced by trap type and pheromone blend. J. Chem. Ecol. 31:2169–2177.
Reddy, G. V. P., Fettköther, R., Noldt, U., and Dettner, K. 2005b. Enhancement of attraction and trap catches of the old-house borer, Hylotrupes bajulus (Coleoptera: Cerambycidae), by combination of male sex pheromone and monoterpenes. Pest Manage. Sci. 61:699–704.
Rodstein, J., Mcelfresh, J. S., Barbour, J. D., Ray, A. M., Hanks, L.M., and Millar, J. G. 2009. Identification and synthesis of a female-produced sex pheromone for the Cerambycid beetle Prionus californicus. J. Chem. Ecol. 35:590–600.
Salunkhe, A. M. and Burkhardt, E. R. 1997. Highly enantioselective reduction of prochiral ketones with N,N-diethylaniline-borane (DEANB) in oxazaborolidine-catalysed reductions. Tetrahed. Lett. 38:1523–1526.
Sas Institute. 2002–2003. Proprietary Software release 9.1 SAS Institute Inc., Cary, NC, USA.
Schimitschek, E. 1929. Tetropium gabrieli Weise und Tetropium fuscum F. Ein beitrag zu ihrer Lebensgeneinsamschaft. Zeits. angew. Entomol. 15:229–334.
Silk, P. J., Sweeney, J. D., Wu, J., Price, J., Gutowski, J. M., and Kettela, E. G. 2007. Evidence for a male-produced pheromone in Tetropium fuscum (F.) and Tetropium cinnamopterum (Kirby) (Coleoptera: Cerambycidae). Naturwissenschaften 94:697–701.
Silk, P. J., Lemay, M. A., Leclair, G., Sweeney, J. D., and Magee, D. 2010 Behavioral and electrophysiological responses of Tetropium fuscum (Coleoptera: Cerambycidae) to spruce volatiles. Environ. Entomol. [in press].
Slessor, K. N., King, G. G. S., Miller, D. R., Winston, M. L., and Cutforth, T. L. 1985. Determination of chirality of alcohol or latent alcohol semiochemicals in individual insects. J. Chem. Ecol. 11:371–374.
Smith, G., and Humble, L. M. 2000. The brown spruce longhorn beetle. Exotic Forest Pest Advisory 5. Natural Resources Canada, Canadian Forest Service. 4 p.
Smith, G., and Hurley, J. E. 2000. First North American record of the Palearctic species Tetropium fuscum (Fabricius) (Coleoptera: Cerambycidae). Coleopt. Bull. 54:540.
Sweeney, J. D., de Groot, P., Macdonald, L., Smith, S., Cocquempot, C., Kenis, M., and Gutowski, J. M. 2004. Host volatile attractants for detection of Tetropium fuscum (F.), Tetropium castaneum (L.), and other longhorned beetles (Coleoptera: Cerambycidae). Environ. Entomol. 33:844–854.
Sweeney, J. D., Gutowski, J. M., Price, J., and de Groot, P. 2006. Effect of semiochemical release rate, killing agent, and trap design on detection of Tetropium fuscum (F.) and other longhorn beetles (Coleoptera: Cerambycidae). Environ. Entomol. 35:645–654.
Symonds, M. R. E., and Elgar, M. A. 2007. The evolution of pheromone diversity. Trends Ecol. Evol. 23:220–228.
Wertheim, B., Van Baalen, E. A., Dicke, M., and Vet, L. E. M. 2005. Pheromone-mediated aggregation in nonsocial arthropods: An evolutionary perspective. Annu. Rev. Entomol. 50:321–346
Zar, J.H. 1999. Biostatistical Analysis. 4th edn. p. 663. Prentice-Hall, Inc., Upper Saddle River, New Jersey.
Zhang, A., Oliver, J.E., Aldrich, J.R., Wang, B., and Mastro, V.C. 2002. Stimulatory beetle volatiles for the Asian longhorned beetle, Anoplophora glabripennis (Motschulsky). Zeitschrift Naturforschung J. Biosci. 57:553–558.
Acknowledgements
We thank the Canadian Food Inspection Agency, Natural Resources Canada—Canadian Forest Service (Forest Invasive Alien Species Fund), and (through the auspices of Spray Efficacy Research Group International) Forest Protection Limited (NB), Ontario Ministry of Natural Resources, and the Nova Scotia Ministry of Natural Resources for generous funding and in-kind support. We thank M. Rhainds, D. Pureswaran, G. LeClair, C. Simpson and two anonymous reviewers for helpful comments on an earlier version of this manuscript, H. Mills for help with synthetic chemistry, M. Lavigne for help with graphics, J. Price, N. Brawn, K. Burgess, A. Doane, B. Guscott, N. Harn, S. Laity, W. MacKay, K. O’Leary, A. Papageorgiou, S. Richards, A. Sharpe, D. Seaboyer, K. Sućko, and T. Walsh for technical assistance, L. Hanks, E. Lacey, J. Millar, and A. Ray for advice, and N. Carter, D. Davies, E. Hurley, and T. Scarr for support. All experiments reported here comply with the laws of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sweeney, J.D., Silk, P.J., Gutowski, J.M. et al. Effect of Chirality, Release Rate, and Host Volatiles on Response of Tetropium fuscum (F.), Tetropium cinnamopterum Kirby, and Tetropium castaneum (L.) to the Aggregation Pheromone, Fuscumol. J Chem Ecol 36, 1309–1321 (2010). https://doi.org/10.1007/s10886-010-9876-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9876-1