Abstract
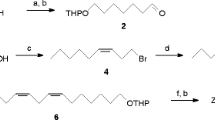
Gas chromatography–mass spectrometry (GC–MS) and GC–electroantennographic detection (EAD) analyses of the sex pheromone extract from a wasp moth, Syntomoides imaon (Lepidoptera: Arctiidae: Syntominae), showed that virgin females produced (Z,Z,Z)-3,6,9-henicosatriene and (Z,Z,Z)-1,3,6,9-henicosatetraene with a trace amount of their C20 analogs. Identification of the chemical structures was facilitated by comparison with authentic standards and the double-bond positions were confirmed by dimethyl disulfide derivatization of monoenes produced by a diimide reduction. In a field test in the Yonaguni-jima Islands, males of the diurnal species were captured in traps baited with a 1:2 mixture of the above-described synthetic C21 polyenes. Lipids were extracted from the abdominal integument and its associated oenocytes and peripheral fat bodies. Following derivatization, fatty acid methyl esters (FAMEs) were fractionated by HPLC equipped with an ODS column, and methyl (Z,Z,Z)-11,14,17-icosatrienoate and (Z,Z,Z)-13,16,19-docosatrienoate were identified by GC–MS. These novel C20 and C22 acid moieties are longer-chain analogs of linolenic acid, (Z,Z,Z)-9,12,15-octadecatrienoic acid. They are presumed to be biosynthetic precursors of the S. imaon pheromone because the C21 trienyl component might be formed by decarboxylation of the C22 acid. On the other hand, the C20 acid, but not the C22 acid, was found in FAMEs of Ascotis selenaria cretacea (Lepidoptera: Geometridae), which secretes C19 pheromone components, (Z,Z,Z)-3,6,9-nonadecatriene and the monoepoxy derivative, indicating that different systems of the chain elongation might play an important role in developing species-specific communication systems mediated with polyunsaturated hydrocarbons and/or epoxy derivatives, components of Type II lepidopteran sex pheromones.




Similar content being viewed by others
References
Ando, T. 2008. Internet database: http://www.tuat.ac.jp/∼antetsu/LepiPheroList.htm.
Ando, T., Hasegawa, Y., and Uchiyama, M. 1986. Separation of lepidopterous sex pheromones by reversed-phase thin layer chromatography and high performance liquid chromatography. Agric. Biol. Chem. 50:2935–2937.
Ando, T., Ohsawa, H., Ueno, T., Kishi, H., Okamura, Y., and Hashimoto, S. 1993. Hydrocarbons with a homoconjugated polyene system and their monoepoxy derivatives: Sex attractants of geometrid and noctuid moths distributed in Japan. J. Chem. Ecol. 19:787–798.
Ando, T., Inomata, S., and Yamamoto, M. 2004. Lepidopteran sex pheromones. Topics Current Chem. 239:51–96.
Ando, T., Kawai, T., and Matsuoka, K. 2008. Epoxyalkenyl sex pheromones produced by female moths in highly evolved groups: Biosynthesis and its endocrine regulation. J. Pestic. Sci. 33:17–20.
Arima, R., Takahara, K., Kadoshima, T., Numazaki, F., Ando, T., Uchiyama, M., Nagasawa, H., and Suzuki, A. 1991. Hormonal regulation of pheromone biosynthesis in the silkworm moth, Bombyx mori. Appl. Entomol. Zool. 26:137–147.
Becker, D., Cyjon, R., Cossé, A., Moore, I., Kimmel, T., and Wysoki, M. 1990. Identification and enantioselective synthesis of (Z,Z)-6,9-cis-3S,4R-epoxynonadecadiene, the major sex pheromone component of Boarmia selenaria. Tetrahedron Lett. 31:4923–4926.
Blomquist, G. J., Nelson, D. R., and de Renobales, M. 1987. Chemistry, biochemistry, and physiology of insect cuticular lipids. Arch. Insect Biochem. Physiol. 6:227–265.
Buser, H.-R., Arn, H., Guerin, P., and Rauscher, S. 1983. Determination of double bond position in mono-unsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Anal. Chem. 55:818–822.
Chertemps, T., Duportets, L., Labeur, C., Ueda, R., Takahashi, K., Saigo, K., and Wicker-Thomas, C. 2007. A female-biased expressed elongase involved in long-chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. U S A 104:4273–4278.
Choi, M.-Y., Lim, H., Park, K. C., Adlof, R., Wang, S., Zhang, A., and Jurenka, R. 2007. Identification and biosynthetic studies of the hydrocarbon sex pheromone in Utetheisa ornatrix. J. Chem. Ecol. 33:1336–1345.
Descoins, C., Lalanne-Cassou, B., Frérot, B., Malosse, C., and Renou, M. 1989. Comparative study of pheromonal secretions produced by arctiid and ctenuchid female moths (insects, Lepidoptera) from the neotropical area. C. R. Acad. Sci., Ser. 3 309:577–581.
Einhorn, J., Boniface, B., Renou, M., and Milat, M.-L. 1984. Study on the sex pheromone of Arctia villica L. (Lepidoptera, Arctiidae). C. R. Acad. Sci., Ser. 3 298:573–576.
El-Sayed, A. M. 2008. Internet database: http://www.pherobase.com/.
Fan, Y., Zurek, L., Dykstra, M. J., and Schal, C. 2003. Hydrocarbon synthesis by enzymatically dissociated oenocytes of the abdominal integument of the German cockroach, Blattella germanica. Naturwissenschaften 90:121–126.
Fujii, T., Suzuki M. G., Kawai, T., Tsuneizumi, K., Ohnishi, A., Kurihara, M., Matsumoto, S., and Ando, T. 2007. Determination of the pheromone producing region that has epoxidation activity in the abdominal tip of the japanese giant looper, Ascotis selenaria cretacea (Lepidoptera: Geometridae). J. Insect Physiol. 53:312–318.
Goller, S., Szöcs, G., Francke, W., and Schulz, S. 2007. Biosynthesis of (3Z,6Z,9Z)-3,6,9-octadecatriene: The main component of the pheromone blend of Erannis bajaria. J. Chem. Ecol. 33:1336–1345.
Jain, S. C., Dussourd, D. E., Conner, W. E., Eisner, T., Guerrero, A., and Meinwald, J. 1983. Polyene pheromone components from an arctiid moth (Utetheisa ornatrix): characterization and synthesis. J. Org. Chem. 48:2266–2270.
Jurenka, R. A., de Renobales, M., and Blomquist, G. J. 1987. De novo biosynthesis of polyunsaturated fatty acid in the cockroach Periplaneta americana. Arch. Biochem. Biophys. 225:184–193.
Jurenka, R. A., Subchev, M., Abad, J.-L., Choi, M.-Y., and Fabrias, G. 2003. Sex pheromone biosynthetic pathway for disparlure in the gypsy moth, Lymantria dispar. Proc. Natl. Acad. Sci. U S A 100:809–914.
Matsuoka, K., Tabunoki, H., Kawai, T., Ishikawa, S., Yamamoto, M., Sato, R., and Ando, T. 2006. Transport of a hydrophobic biosynthetic precursor by lipophorin in the hemolymph of geometrid female moth which secretes an epoxyalkenyl sex pheromone. Insect Biochem. Mol. Biol. 36:576–583.
Miyamoto, T., Yamamoto, M., Ono, A., Ohtani, K., and Ando, T. 1999. Substrate specificity of the epoxidation reaction in sex pheromone biosynthesis of the Japanese giant looper (Lepidoptera: Geometridae). Insect Biochem. Mol. Biol. 29:63–69.
Naka, H., Nakazawa, T., Sugie, M., Yamamoto, M., Horie, Y., Wakasugi, R., Arita, Y., and Ando, T. 2006. Synthesis and characterization of 3,13-and 2,13-octadecadienyl compounds for identification of the sex pheromone secreted by a clearwing moth, Nokona pernix. Biosci. Biotechnol. Biochem. 70:508–516.
Pohnert, G. and Boland,W. 2000. High efficient one-pot double-Wittig approach to unsymmetrical (1Z,4Z,7Z)-homoconjugated trienes. Eur. J. Org. Chem. 1821–1826.
Romer, F. 1991. The oenocytes of insects: Differentiation, changes during molting, and heir involvement in the secretion of molting hormone, pp. 532–567, in A.P. Gupta (ed.). Morphogenetic Hormones of Arthropods: Roles in Histogenesis, Organogenesis, and Morphogenesis Rutgers University Press, New Brunswick, NJ.
Rule, G. S., and Roelofs, W. L. 1989. Biosynthesis of sex pheromone components from linolenic acid in arctiid moths. Arch. Insect Biochem. Physiol. 12:89–97.
Struble, D. L., and Arn, H. 1984. Combined gas chromatography and electroantennogram recording of insect olfactory responses, pp. 161–178, in H.E. Hummel, and T.A. Miller (eds.). Techniques in Pheromone Research Springer-Verlag, New York, NY.
Tillman-Wall, J. A., Vanderwel, D., Kuenzli, M. E., Reitz, R. C., and Blomquist, G. J. 1992. Regulation of sex pheromone biosynthesis in the housefly, Musca domestica: Relative contribution of the elongation and reductive steps. Arch. Biochem. Biophys. 299:92–99.
Vaz, A. H., Jurenka, R. A., Blomquist, G. J., and Reitz, R. C. 1988. Tissue and chain length specificity of the fatty acyl-CoA elongation system in the American cockroach. Arch. Biochem. Biophys. 267:551–557.
Wei, W., Miyamoto, T., Endo, M., Murakawa, T., Pu, G.-Q., and Ando, T. 2003. Polyunsaturated hydrocarbons in the hemolymph: Biosynthetic precursors of epoxy pheromones of geometrid and arctiid moths. Insect Biochem. Mol. Biol. 33:397–405.
Yamamoto, M., Kamata, T., Do, N. D., Adachi, Y., Kinjo, M., and Ando, T. 2007. A novel lepidopteran sex pheromone produced by females of a Lithosiinae species, Lyclene dharma dharma, in the family of Arctiidae. Biosci. Biotechnol. Biochem. 71:2860–2863.
Yamamoto, M., Yamakawa, R., Oga, T., Takei, Y., Kinjo, M., and Ando, T. 2008. Synthesis and chemical characterization of hydrocarbons with a 6,9,11-, 3,6,9,11-, or 1,3,6,9-polyene system, pheromone candidates in Lepidoptera. J. Chem. Ecol. 34:1057–1064.
Yamaoka, R., Fukami, H., and Ishii, S. 1976. Isolation and identification of the female sex pheromone of the potato tuberworm moth, Phthorimaea operculella (Zeller). Agric. Biol. Chem. 40:1971–1977.
Zhu, J.-W., Löfstedt, C., Philipp, P., Francke, W., Tammaru, T., and Haukioja, E. 1995. A sex pheromone component to the Geometridae identified from Epirrita autumnata. Entomol. Exp. Appl. 75:159–164.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuoka, K., Yamamoto, M., Yamakawa, R. et al. Identification of Novel C20 and C22 Trienoic Acids from Arctiid and Geometrid Female Moths that Produce Polyenyl Type II Sex Pheromone Components. J Chem Ecol 34, 1437–1445 (2008). https://doi.org/10.1007/s10886-008-9530-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9530-3