Abstract
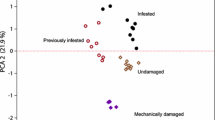
Two issues have hindered the understanding of the ecology and evolution of volatile-mediated tritrophic interactions: few studies have addressed noncrop systems; and few statistical techniques have been applied that are suitable for the analysis of complex volatile blends. In this paper, we addressed both of these issues by studying the noncrop system involving the plant Centaurea nigra, the specialist aphid Uroleucon jaceae, and the parasitoid Aphidius funebris. In a Y-tube olfactometer, A. funebris was attracted to the odor from undamaged C. nigra, but preferred the plant–host complex (PHC) after 3 d of feeding by 200 U. jaceae over the undamaged plant, but not after three or 5 d of feeding by 50 U. jaceae. When aphids were removed, the initial preference for the damaged plant remained, but the final preference was not greater than for the undamaged plant. No qualitative differences were detected between the headspaces of C. nigra and the C. nigra–U. jaceae PHC. For quantitative analysis, we used a compositional approach, which treats each compound produced as part of a blend, and not as a compound released in isolation, thus allowing analysis of the relative contribution of each compound to the blend as a whole. With this approach, subtle increases and decreases of some green leaf volatiles and monoterpenoids on the third day of aphid infestation were detected. Mechanically damaged C. nigra had a volatile profile that differed from undamaged C. nigra and the PHC. One and 10 ng of (Z)-3-hexenyl acetate, and 10 or 100 ng of 6-methyl-5-hepten-2-one were attractive to the parasitoid when placed in solution on filter paper. A. funebris appears to be using a combination of chemical cues to locate host-infested plants.



Similar content being viewed by others
References
Aitchison, J. 1986. The Statistical Analysis of Compositional Data. Chapman & Hall, Inc., Bristol.
Bernays, E. A. and Funk, D. J. 2000. Electrical penetration graph analysis reveals population differentiation of host-plant probing behaviors within the aphid species Uroleucon ambrosiae. Entomol. Exp. Appl. 97:183–191.
Blackman, R. L. and Eastop, V. F. 2000. Aphids on the World’s Crops. John Wiley and Sons Ltd., New York.
Bostock, R. M. 2005. Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu. Rev. Phytopathol. 43:545–580.
Bronstein, J. L. and Barbosa, P. 2002. Multitrophic/multispecies mutualistic interactions: the role of non-mutualists in shaping and mediating mutualisms. in T. Tscharntke and B. A. Hawkins (eds.). Multitrophic level interactions pp. 44–66. Cambridge: Cambridge University Press.
Bukovinszky, T., Gols, R., Posthumus, M. A., Vet, L. E. M., and Van Lenteren, J. C. 2005. Variation in plant volatiles and attraction of the parasitoid Diadegma semiclausum (Hellén). J. Chem. Ecol. 31:461–480.
Colazza, S., Fucarino, A., Peri, E., Salerno, G., Conti, E., and Bin, F. 2004a. Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J. Exp. Biol. 207:47–53.
Colazza, S., Mcelfresh, J. S., and Millar, J. G. 2004b. Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid Trissolcus basalis. J. Chem. Ecol. 30:945–964.
De Moraes, C. M., Lewis, W. J., Paré, P. W., Alborn, H. T., and Tumlinson, J. H. 1998. Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573.
Dicke, M., Van Poecke, R. M. P., and De Boer, J. G. 2003. Inducible defense of plants: from mechanisms to ecological functions. Basic Appl. Ecol. 4:27–42.
Du, Y.-J., Poppy, G. M., and Powell, W. 1996. Relative importance of semiochemicals from first and second trophic levels in host foraging behavior of Aphidius ervi. J. Chem. Ecol. 22:1591–1605.
Du, Y.-J., Poppy, G. M., Powell, W., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1998. Identification of semiochemicals released during aphid feeding that attract the parasitoid Aphidius ervi. J. Chem. Ecol. 24:1355–1368.
Fäldt, J., Arimura, G.-I., Gershenzon, J., Takabayashi, J., and Bohlmann, J. 2003. Functional identification of AtTPS03 as (E)-β-ocimene synthase: a monoterpene synthase catalyzing jasmonate- and wound-induced volatile formation in Arabidopsis thaliana. Planta 216:745–751.
Feeny, P. 1976. Plant apparency and chemical defense. Recent Adv. Phytochem. 10:1–40.
Geervliet, J. B. F., Verdel, M. S. W., Snellen, H., Schaub, J., Dicke, M., and Vet, L. E. M. 2000. Coexistence and niche segregation by field populations of the parasitoids Cotesia glomerata and C. rubecula in the Netherlands: predicting field performance from laboratory data. Oecologia 124:55–63.
Gouinguené, S., Degen, T., and Turlings, T. C. J. 2001. Variability in herbivore-induced odor emissions among maize cultivars and their wild ancestors. Chemoecology 11:9–16.
Gouinguené, S., Pickett, J. A., Wadhams, L. J., Birkett, M. A., and Turlings, T. C. J. 2005. Antennal electrophysiological responses of three parasitic wasps to caterpillar-induced volatiles from maize (Zea mays mays), cotton (Gossypium herbaceum), and cowpea (Vigna unguiculata). J. Chem. Ecol. 31:1023–1038.
Greenhouse, S. W. and Geisser, S. 1959. On methods in the analysis of profile data. Psychometrika 24:95–112.
Guerrieri, E., Poppy, G. M., Powell, W., Tremblay, E., and Penacchio, F. 1999. Induction and systemic release of herbivore-induced plant volatiles mediating in-flight orientation of Aphidius ervi. J. Chem. Ecol. 25:1247–1261.
Heidel, A. J. and Baldwin, I. T. 2004. Microarray analysis of salicylic acid- and jasmonic acid-signalling in responses of Nicotiana attenuata to attack by insects from multiple feeding guilds. Plant Cell Environ. 27:1362–1373.
Hoballah, M. E. and Turlings, T. C. J. 2005. The role of fresh versus old leaf damage in the attraction of parasitic wasps to herbivore-induced maize volatiles. J. Chem. Ecol. 31:2003–2018.
Kaloshian, I. and Walling, L. L. 2005. Hemipterans as plant pathogens. Annu. Rev. Phytopathol. 43:491–521.
Kessler, A. and Baldwin, I. T. 2002. Plant responses to insect herbivores: the emerging molecular analysis. Annu. Rev. Plant Biol. 53:299–328.
Lo Pinto, M., Wajnberg, E., Colazza, S., Curty, C., and Fauvergue, X. 2004. Olfactory response of two aphid parasitoids, Lysiphlebus testaceipes and Aphidius colemani, to aphid-infested plants from a distance. Entomol. Exp. Appl. 110:159–164.
Martinez De Ilarduya, O., Xie, Q., and Kaloshian, I. 2003. Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol. Plant-Microb. Interact. 16:699–708.
Mattiacci, L., Ambühl Rocca, B., Scascighini, N., D’alessandro, M., Hern, A., and Dorn, S. 2001. Systemically induced plant volatiles emitted at the time of “danger”. J. Chem. Ecol. 27:2233–2252.
Miles, P. W. 1999. Aphid saliva. Biol. Rev. 74:41–85.
Moraes, M. C. B., Laumann, R., Sujii, E. R., Pires, C., and Borges, M. 2005. Induced volatiles in soybean and pigeon pea plants artificially infested with the neotropical brown stink bug, Euschistus heros, and their effect on the egg parasitoid, Telenomus podisi. Entomol. Exp. Appl. 115:227–237.
Moran, N. A. 1986. Morphological adaptation to host plants in Uroleucon (Homoptera: Aphididae). Evolution 40:1044–1050.
Moran, P. J. and Thompson, G. A. 2001. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 125:1074–1085.
Ngi-Song, A. J., Njagi, P. G. N., Torto, B., and Overholt, W. A. 2000. Identification of behaviorally active components from maize volatiles for the stemborer parasitoid Cotesia flavipes Cameron (Hymenoptera: Braconidae). Insect Sci. Appl. 20:181–189.
Paré, P. W. and Tumlinson, J. H. 1997. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 114:1161–1167.
Pareja, M. F. 2006. Tritrophic interactions and chemical ecology of a non-crop plant-aphid-parasitoid system. PhD dissertation. University of Reading, Reading, UK.
Pauw, B. and Memelink, J. 2004. Jasmonate-responsive gene expression. J. Plant Growth Regul. 23:200–210.
Payne, R. W., Harding, S. A., Murray, D. A., Soutar, D. M., Baird, D. B., Welham, S. J., Kane, A. F., Gilmour, A. R., Thompson, R., Webster, R., et al. 2005. GenStat Release 8 Reference Manual. Part 3: Procedure Library PL16. VSN International, Oxford.
Powell, W., Pennacchio, F., Poppy, G. M., and Tremblay, E. 1998. Strategies involved in the location of hosts by the parasitoid Aphidius ervi Haliday (Hymenoptera: Braconidae: Aphidiinae). Biol. Control 11:104–112.
Roßbach, A., Löhr, B., and Vidal, S. 2005. Generalism vesus specialism: responses of Diadegma mollipla (Holmgren) and Diadegema semiclausum (Hellen), to the host shift of the diamondback moth (Plutella xylostella L.) to peas. J. Insect Behav. 18:491–503.
Röse, U. S. R., Lewis, W. J., and Tumlinson, J. H. 1998. Specificity of systemically released cotton volatiles as attractants for specialist and generalist parasitic wasps. J. Chem. Ecol. 24:303–319.
Shiojiri, K., Takabayashi, J., Yano, S., and Takafuji, A. 2001. Infochemically mediated tritrophic interaction webs on cabbage plants. Popul. Ecol. 43:23–29.
Shiojiri, K., Ozawa, R., Matsui, K., Kishimoto, K., Kugimiya, S., and Takabayashi, J. 2006. Role of the lipoxygenase/lyase pathway of host-food plants in the host searching behavior of two parasitoid species, Cotesia glomerata and Cotesia plutellae. J. Chem. Ecol. 32:969–979.
Smid, H. M., Van Loon, J. J. A., Posthumus, M. A., and Vet, L. E. M. 2002. GC-EAG-analysis of volatiles from Brussels sprouts plants damaged by two species of Pieris caterpillars: olfactory receptive range of a specialist and a generalist parasitoid wasp species. Chemoecology 12:169–176.
Takabayashi, J., Noda, T., and Takahashi, S. 1991. Plants produce attractants for Apanteles kariyai, a parasitoid of Pseudaletia separata; cases of ‘communication’ and ‘misunderstanding’ in parasitoid-plant interactions. Appl. Entomol. Zool. 26(2):237–243.
Tjallingii, W. F. 2006. Salivary secretions by aphids interacting with proteins of phloem wound responses. J. Exp. Bot. 57:739–745.
Turlings, T. C. J., Tumlinson, J. H., Eller, F. J., and Lewis, W. J. 1991. Larval-damaged plants: source of volatile synomones that guide the parasitoid Cotesia marginiventris to the micro-habitat of its hosts. Entomol. Exp. Appl. 58:75–82.
Turlings, T. C. J., Lengwiler, U. B., Bernasconi, M. L., and Weschler, D. 1998. Timing of induced volatile emissions in maize seedlings. Planta 207:146–152.
Van Poecke, R. M. P., Roosjen, M., Pumarino, L., and Dicke, M. 2003. Attraction of the specialist parasitoid Cotesia rubecula to Arabidopsis thaliana infested by host or non-host herbivore species. Entomol. Exp. Appl. 107:229–236.
Vet, L. E. M. 1996. Parasitoid foraging: the importance of variation in individual behaviour for population dynamics. in R. B. Floyd, A. W. Sheppard, and P. J. De Barro (eds.). Frontiers of population ecology pp. 245–256. Melbourne: CSIRO Publishing.
Vet, L. E. M. 1999. From chemical to population ecology: infochemical use in an evolutionary context. J. Chem. Ecol. 25:31–49.
Vet, L. E. M. and Dicke, M. 1992. Ecology of infochemical use by natural enemies in a tritophic context. Annu. Rev. Entomol. 37:141–172.
Vinson, S. B. 1976. Host selection by insect parasitoids. Annu. Rev. Entomol. 21:109–134.
Völkl, W., Kranz, P., Weisser, W., and Hübner, G. 1995. Patch time allocation and resource exploitation in aphid primary parasitoids and hyperparasitoids searching simultaneously within aphid colonies. J. Appl. Entomol. 119:399–404.
Walling, L. L. 2000. The myriad plant responses to herbivores. J. Plant Growth Regul. 19:195–216.
Weisser, W. W. 1995. Within-patch foraging behaviour of the aphid parasitoid Aphidius funebris: plant architecture, host behaviour, and individual variation. Entomol. Exp. Appl. 76:133–141.
Whitman, D. W. and Eller, F. J. 1992. Orientation of Microplitis croceipes (Hymenoptera: Braconidae) to green leaf volatiles: dose–response curves. J. Chem. Ecol. 18:1743–1753.
Will, T. and Van Bel, A. J. E. 2006. Physical and chemical interactions between aphids and plants. J. Exp. Bot. 57:729–737.
Acknowledgements
We are grateful to K. Chamberlain, L. Wadhams, and M. Borges for providing help with the chemical analyses, J. Aldrich for providing synthetic standards, V. Brown for advice on the experiments, G. Piaggio for statistical advice and comments on previous versions of the manuscript, and M. Torrance and K. Plumb for help in rearing. We thank two anonymous reviewers and M. Hilker for valuable comments during the review process. This work was funded by a Lawes Trust–University of Reading award to MP. Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC) of the United Kingdom.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pareja, M., Moraes, M.C.B., Clark, S.J. et al. Response of the Aphid Parasitoid Aphidius funebris to Volatiles from Undamaged and Aphid-infested Centaurea nigra . J Chem Ecol 33, 695–710 (2007). https://doi.org/10.1007/s10886-007-9260-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9260-y