Abstract
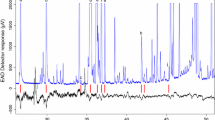
The stemborers Chilo partellus and Busseola fusca are major pests of subsistence cereal farming in Africa. Volatiles released by two cultivated hosts, sorghum and maize (Sorghum bicolor and Zea mays), and two wild grass hosts, Pennisetum purpureum and Hyparrhenia tamba, were collected by air entrainment. Electrophysiologically active components in these samples were detected by coupled gas chromatography-electroantennography (GC-EAG), and the active peaks identified by gas chromatography-mass spectrometry. A total of 41 compounds were identified from the four plant species, all of which, as well as two unidentified compounds, elicited an electrophysiological response from one or both of the stemborers. The compounds included a number of green leaf volatiles and other aliphatic aldehydes, ketones, and esters, mono- and sesquiterpenoids, and some aromatic compounds. EAG studies with authentic samples, conducted at two discriminating doses for all compounds, and dose–response curves for 14 of the most highly EAG-active compounds, showed significant differences in relative responses between species. The compounds that elicited large responses in both species of moths included linalool, acetophenone, and 4-allylanisole, while a number of compounds such as the aliphatic aldehydes octanal, nonanal, and decanal elicited a large response in B. fusca, but a significantly smaller response in C. partellus. Furthermore, the wild hosts produced higher levels of physiologically active compounds compared with either of the cultivated hosts. These differences are discussed in relation to the differential attraction/oviposition of the two stemborers observed in the field and, particularly for eastern African small-scale farming systems, in the context of using a push–pull strategy for their control.




Similar content being viewed by others
References
Agelopoulos, N. G., Hooper, A. M., Maniar, S. P., Pickett, J. A., and Wadhams, L. J. 1999. A novel approach for isolation of volatile chemicals released by individual leaves of a plant in situ. J. Chem. Ecol. 25:1411–1425.
Bernasconi, M. L., Turlings, T. C. J., Ambrosetti, L., Bassetti, P., and Dorn, S. 1998. Herbivore-induced emissions of maize volatiles repel the corn leaf aphid, Rhopalosiphum maidis. Entomol. Exp. Appl. 87:133–142.
Bernays, E. A. and Chapman, R. F. 1994. Host Plant Selection by Phytophagous Insects. Chapman and Hall.
Birkett, M. A., Chamberlain, K., Guerrieri, E., Pickett, J. A., Wadhams, L. J., and Yasuda, T. 2003. Volatiles from whitefly-infested plants elicit a host-locating response in the parasitoid, Encarsia formosa. J. Chem. Ecol. 29:1589–1600.
Bruce, T. J. A., Wadhams, L. J., and Woodcock, C. M. 2005. Insect host location: A volatile situation. Trends Plant Sci. 10:269–274.
Chamberlain, K., Khan, Z. R., Pickett, J. A., Toshova, T., and Wadhams, L. J. 2006. Diel periodicity in the production of green leaf volatiles by wild and cultivated host plants of stemborer moths Busseola fusca and Chilo partellus. J. Chem. Ecol. 32(3):565–577.
Chou, T., Tso, H., and Chang, L. 1984. Stereoselective one-step syntheses of trans-ocimene and -farnesene. J. Chem. Soc. Chem. Comm. 1323.
Degen, T., Dillmann, C., Marion-Poll, F., and Turlings, T. C. J. 2004. High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol. 135:1928–1938.
Dethier, V. G. 1982. Mechanism of host-plant recognition. Entomol. Exp. Appl. 31:49–56.
Gaoni, Y. 1977. Lithium aluminium hydride promoted extrusion of SO2 from sulfolenes and sulfolene-adducts. 1,3-Dienes, 3-chloro- and 3,3-dichloro-1,4-dienes and skipped polyenes. Tetrahedron Lett. 11:947–950.
Gouinguené, S., Degen, T., and Turlings, T. C. J. 2001. Variability in herbivore-induced odour emissions among corn cultivars and their wild ancestors (teosinte). Chemoecology 11:9–16.
Kang, S. K. and Kim, S. G. 1986. A practical synthesis of (E)-β-farnesene, the alarm pheromone of aphids. Bull. Korean Chem. Soc. 7:157–159.
Khan, Z. R. and Pickett, J. A. 2004. The ‘push–pull’ strategy for stemborer management: A case study in exploiting biodiversity and chemical ecology, pp. 155–164, in G. M. Gurr, S. D. Wratten, and M. A. Altieri (eds.). Ecological Engineering for Pest Management. CABI, Oxford, UK.
Khan, Z. R., Chiliswa, P., Ampong-Nyarko, K., Smart, L. E., Polaszek, A., Wandera, J., and Mulaa, M. A. 1997a. Utilisation of wild gramineous plants for management of cereal stemborers in Africa. Insect Sci. Appl. 17:143–150.
Khan, Z. R., Ampong-Nyarko, K., Chiliswa, P., Hassanali, A., Kimani, S., Lwande, W., Overholt, W. A., Pickett, J. A., Smart, L. E., Wadhams, L. J., and Woodcock, C. M. 1997b. Intercropping increases parasitism of pests. Nature 388:631–632.
Khan, Z. R., Pickett, J. A., Van Den Berg, J., Wadhams, L. J., and Woodcock, C. M. 2000. Exploiting chemical ecology and species diversity: Stem borer and Striga control for maize and sorghum in Africa. Pest Manag. Sci. 56:957–962.
Khan, Z. R., Pickett, J. A., Wadhams, L., and Muyekho, F. 2001. Habitat management strategies for the control of cereal stemborers and Striga in maize in Kenya. Insect Sci. Appl. 21:375–380.
Khan, Z. R., Mohamed, H. M., Overholt, W. A., and Elizabeth, D. K. 2004. Behaviour and biology of Chilo partellus (Lepidoptera: Pyralidae) on maize and wild gramineous plants. Int. J. Trop. Insect Sci. 24:287–297.
Khan, Z. R., Midega, C. A. O., Hutter, N. J., Wilkins, R. M., and Wadhams, L. J. 2006. Assessment of the potential of Napier grass (Pennisetum purpureum) varieties as trap plants for management of Chilo partellus. Entomol. Exp. Appl. 119:15–22.
Loughrin, J. H., Manukian, A., Heath, R. R., Turlings, T. C. J., and Tumlinson, J. H. 1994. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plants. Proc. Natl. Acad. Sci. USA 91:11836–11840.
Maddrell, S. H. 1969. Secretion by Malpighian tubules of Rhodnius. Movements of ions and water. J. Exp. Bot. 51:71–78.
Maurer, B., Hauser, A., and Froidevaux, J. C. 1986. (E)-4,8-Dimethyl-1,3,7-nonatriene and (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene, two unusual hydrocarbons from cardamon oil. Tetrahedron Lett. 27:2111–2112.
Ndemah, R., Gounou, S., and Schulthess, F. 2002. The role of wild grasses in the management of lepidopterous stem-borers on maize in the humid tropics of Western Africa. Bull. Entomol. Res. 92:507–519.
Ngi-Song, A. J., Njagi, P. G. N., Torto, B., and Overholt, W. A. 2000. Identification of behaviourally active components of maize volatiles for the stemborer parasitoid Cotesia flavipes Cameron (Hymenoptera: Braconidae). Insect Sci. Appl. 20(3):181–189.
Päts, P. 1991. Activity of Chilo partellus (Lepidoptera: Pyralidae): Eclosion, mating and oviposition time. Bull. Entomol. Res. 81:93–96.
Pickett, J. A. 1990. Gas chromatography-mass spectrometry (GC-MS) in insect pheromone identification: Three extreme case histories, pp. 299–309, in A.R. McCaffery and I.D. Wilson (eds.). Chromatography and Isolation of Insect Hormones and Pheromones. Plenum, New York.
Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1998. Insect supersense: Mate and host location by insects as model systems for exploiting olfactory interactions. The Biochemist 20:8–13.
Rebe, M., Van Den Berg, J., and McGeoch, M. A. 2004. Colonisation of cultivated and indigenous graminaceous host plants by Busseola fusca (Fuller) (Lepidoptera: Noctuidae) and Chilo partellus (Swinhoe) (Lepidoptera: Crambidae) under field conditions. Afr. Entomol. 12:187–199.
Takabayashi, J., Takanashi, S. Dicke, M., and Posthumus, M. A. 1995. Developmental stage of herbivore Pseudeletia separata affects production of herbivore induced synomone by corn plants. J. Chem. Ecol. 21:273–287.
Turlings, T. C. J., Tumlinson, J. H., and Lewis, W. J. 1990. Exploitation of herbivore-induced plant odours by host-seeking parasitic wasps. Science (Washington) 250:1251–1253.
Turlings, T. C. J., Tumlinson, J. H., Heath, R. R., Proveaux, A. T., and Doolittle, R. E. 1991. Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J. Chem. Ecol. 17:2235–2251.
Turlings, T. C. J., Loughrin, J. H., Mccall, P. J., Röse, U. S. R., Lewis, W. J., and Tumlinson, J. H. 1995. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl. Acad. Sci. USA 92:4169–4174.
Turlings, T. C. J., Bernasconi, M. L., Bertossa, R., Bigler, F., Caloz, G., and Dorn, S. 1998a. The induction of volatile emissions in maize by three herbivore species with different feeding habits: possible consequences for their natural enemies. Biol. Control 11:122–129.
Turlings, T. C. J., Lengwiler, U. B., Bernasconi, M. L., and Wechsler, D. 1998b. Timing of induced volatile emissions in corn seedlings. Planta 207:146–152.
Visser, J. H. 1986. Host odor perception in phytophagous insects. Annu. Rev. Entomol. 31:121–144.
Visser, J. H. 1988. Host-plant finding by insects—orientation, sensory input and search patterns. J. Insect Physiol. 34:259–268.
Wadhams, L. J. 1990. The use of coupled gas chromatography-electrophysiological techniques in the identification of insect pheromones, pp. 289–298, in A. R. McCaffery and I. D. Wilson (eds.) Chromatography and Isolation of Insect Hormones and Pheromones. Plenum, New York.
Wadhams, L. J., Angst, M. E., and Blight, M. M. 1982. Responses of the olfactory receptors of Scolytus scolytus (F.) (Coleoptera: Scolytidae) to the stereoisomers of 4-methyl-3-heptanol. J. Chem. Ecol. 8:477–492.
Acknowledgments
Teodora Toshova was the recipient of a Royal Society Fellowship. Rothamsted Research received grant-aided support from the Biotechnology and Biological Research Council of the UK. The work was also supported by the UK Department for the Environment, Food, and Rural Affairs (DEFRA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Birkett, M.A., Chamberlain, K., Khan, Z.R. et al. Electrophysiological Responses of the Lepidopterous Stemborers Chilo partellus and Busseola fusca to Volatiles from Wild and Cultivated Host Plants. J Chem Ecol 32, 2475–2487 (2006). https://doi.org/10.1007/s10886-006-9165-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9165-1