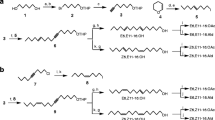
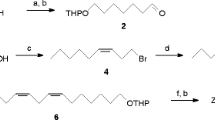
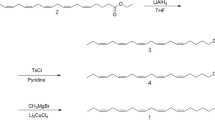
Abstract
Gas chromatography–electroantennographic detection analysis of sex pheromone gland extracts of the common forest looper Pseudocoremia suavis (Lepidoptera: Geometridae), a polyphagous defoliator of introduced Pinaceae and many New Zealand trees, revealed four compounds that elicited antennal responses. The two major active compounds (6Z)-cis-9,10-epoxynonadec-6-ene and (3Z,6Z)-cis-9,10-epoxynonadeca-3,6-diene were identified by comparison with known standards. Of the two minor active compounds, one was tentatively identified as (3Z,6Z)-cis-9,10-epoxyhenicosa-3,6-diene, whereas the other could not be identified because of insufficient amounts in extracts. (6Z)-cis-9,10-Epoxynonadec-6-ene, (3Z,6Z)-cis-9,10-epoxynonadeca-3,6-diene, and (3Z,6Z)-cis-9,10-epoxyhenicosa-3,6-diene were present in P. suavis gland extracts from Eyrewell Forest, a Pinus radiata plantation in the South Island of New Zealand, in a ratio of 35:65:5, respectively. Trapping trials in Eyrewell Forest established that (6Z)-cis-9,10-epoxynonadec-6-ene attracted male P. suavis. However, addition of (3Z,6Z)-cis-9,10-epoxyhenicosa-3,6-diene to the lure at <10% of (6Z)-cis-9,10-epoxynonadec-6-ene reduced capture of male moths, suggesting that one of its enantiomers was acting as a behavioral antagonist. During January–March of 2005, a blend trial involving single, binary, and ternary mixtures of the three components at Eyrewell Forest and at three other sites (two in the South Island and one in the North Island) revealed the existence of a second taxon of P. suavis at the three additional sites that was attracted to lures containing (3Z,6Z)-cis-9,10-epoxynonadeca-3,6-diene, either singly or in binary and ternary mixtures with (6Z)-cis-9,10-epoxynonadec-6-ene and (3Z,6Z)-cis-9,10-epoxyhenicosa-3,6-diene. This second taxon was not attracted to lures loaded solely with (6Z)-cis-9,10-epoxynonadec-6-ene.




Similar content being viewed by others
References
Alma, P. J. 1977. Selidosema [= Pseudocoremia] suavis (Butler) (Lepidoptera: Geometridae). Forest and Timber Insects in New Zealand, No. 11. Forest Research Institute, Rotorua, pp. 4.
Ando, T., Ohsawa, H., Ueno, T., Kishi, H., Okamura, Y., and Hashimoto, S. 1993. Hydrocarbons with a homoconjugated polyene system and their monoepoxy derivatives: Sex attractants of geometrid and noctuid moths distributed in Japan. J. Chem. Ecol. 19:787–798.
Ando, T., Kishi, H., Akashio, N., Qin, X., Saito, N., Abe, H., and Hashimoto, S. 1995. Sex attractants of geometrid and noctuid moths: Chemical characterization and field test of monoepoxides of 6,9-dienes and related compounds. J. Chem. Ecol. 21:299–311.
Ando, T., Inomata, S., and Yamamoto, M. 2004. Lepidopteran sex pheromones. Top. Curr. Chem. 239:51–96.
Berndt, L., Brockerhoff, E. G., Jactel, H., Weis, T., and Beaton, J. 2004. Biology and rearing of Pseudocoremia suavis, an endemic looper (Lepidoptera: Geometridae) with a history of outbreaks on exotic conifers. N. Z. Entomol. 27:73–82.
Clare, G., Suckling, D. M., Bradley, S. J., Walker, J. T. S., Shaw, P. W., Daly, J. M., McLaren, G. F., and Wearing, C. H. 2000. Pheromone trap colour determines catch of non target insects. N. Z. Plant Prot. 53:216–220
Dugdale, J. S. 1958. Structural characters of the larva of Selidosema [= Pseudocoremia] suavis (Butler) (Lepidoptera: Geometridae, Subfamily Ennominae). N. Z. Entomol. 2:24–33.
Dugdale, J. S. 1989. New Zealand Lepidoptera; basic biogeography. N.Z. J. Zool. 16:679–687.
El-Sayed, A. M. 2006. The Pherobase: Database of Insect Pheromones and Semiochemicals. http://www.pherobase.com.
El-Sayed, A. M., Gibb, A. R., Suckling, D. M., Bunn, B., Fielder, S., Comeskey, D., Manning, L. A., Foster, S. P., Morris, B. D., Ando, T., and Mori, K. 2005. Identification of sex pheromone components of the painted apple moth: a tussock moth with a thermally labile pheromone component. J. Chem. Ecol. 31:633–657.
Foster, S. P., Dugdale, J. S., and White, C. S. 1991. Sex pheromones and the status of the greenheaded and the brownheaded leafroller moths in New Zealand. N.Z. J. Zool. 18:63–74.
Frérot, B. and Foster, S. P. 1991. Sex pheromone evidence for two distinct taxa within Graphania mutans (Walker). J. Chem. Ecol. 17:2077–2093.
Kováts, E. 1965. Gas chromatographic characterization of organic substances in the retention index system, pp. 229–247, in J. C. Giddings and R. A. Keller (eds.). Advances in Chromatography, Vol. 1. Edward Arnold Ltd., London.
Marques, F. A., McElfresh, J. S., and Millar, J. G. 2000. Kováts retention indexes of monounsaturated C12, C14, and C16 alcohols, acetates and aldehydes commonly found in lepidopteran pheromone blends. J. Braz. Chem. Soc. 11:592–599.
Millar, J. G. 2000. Polyene hydrocarbons and epoxides: a second major class of lepidopteran sex attractant pheromones. Annu. Rev. Entomol. 45:575–604.
Millar, J. G., Giblin, M., Barton, D., and Underhill, E. W. 1991. Synthesis and field screening of chiral monounsaturated epoxides as lepidopteran sex attractants and sex pheromone components. J. Chem. Ecol. 17:911–929.
Mori, K. 1998. Chirality and insect pheromones. Chirality 10:578–586.
Nuttall, M. 1983. Pseudocoremia fenerata (Felder) (Lepidoptera, Geometridae): A native looper. Forest and Timber Insects in New Zealand, No. 56. Forest Research Institute, Rotorua, pp. 4.
SAS Institute Inc. 1998. Statview. SAS Institute Inc., Cary, NC.
Soulie, J. B. and Lallemand, Y. 1995. Access to unsaturated chiral epoxides. Part II. Synthesis of a component of the sex pheromone of Phragmatobia fuliginosa. Tetrahedron: Asymmetry 6:625–636.
Steck, W. F., Underhill, E. W., Bailey, B. K., and Chisholm, M. D. 1982. (Z)-7-Tetradecenal, a seasonally dependent sex pheromone of the W-marked cutworm, Spaelotis clandestina (Harris) (Lepidoptera: Noctuidae). Environ. Entomol. 11:1119–1122.
Stephens, A. E. A. 2001. Pseudocoremia (Lepidoptera: Geometridae: Ennominae) Systematics, biogeography and host plant associations. MSc thesis, Victoria University of Wellington, New Zealand.
Suckling, D. M. and Karg, G. 2000. Pheromones and semiochemicals, pp. 63–99, in J. Rechcigl and N. Rechcigl (eds.). Biological and Biotechnical Control of Insect Pests. CRC Press, Boca Raton, FL.
Szöcs, G., Tóth, M., Francke, W., Schmidt, F., Philipp, P., König, W. A., Mori, K., Hansson, B. S., and Löfstedt, C. 1993. Species discrimination in five species of winter-flying geometrids (Lepidoptera) based on chirality of semiochemicals and flight season. J. Chem. Ecol. 19:2721–2735.
White, T. R. C. 1974. A hypothesis to explain outbreaks of looper caterpillars, with special reference to populations of Selidosema [= Pseudocoremia] suavis in a plantation of Pinus radiata in New Zealand. Oecologia 16:279–301.
Windholz, M. (ed.) 1983. The Merck Index. Tenth Edition. Merck & Co., Inc.
Zhang, Z., Wang, Z., Wang, Y., Liu, H., Lei, G., and Shi, M. 1999. A facile synthetic method for chiral 1,2-epoxides and the total synthesis of chiral pheromone epoxides. Tetrahedron: Asymmetry 10:837–840.
Acknowledgments
We thank John Allen (HortResearch, Palmerston North) and Diane Steward and Robert Franich (Forest Research, Rotorua) for mass spectrometry, and Carter Holt Harvey, Selwyn Plantation Board, and John and Jenny Taylor for access to trapping sites. Tetsu Ando, Tokyo University of Agriculture and Technology, Japan, provided samples of 3Z,6Z-cis-9,10-epo-21Hy, (3Z,6Z)-9S,10R-epoxyhenicosa-3,6-diene, and (3Z,6Z)-9R,10S-epoxyhenicosa-3,6-diene. Technical assistance in the field was provided by Paula Thompson (HortResearch, Lincoln) and Belinda Gresham (Ensis, Rotorua). Thanks are also due to Barry Donovan for placement and checking of a trap at Whataroa and to John Dugdale, Andréa Stephens, and David Logan for commenting on earlier versions of the manuscript. Funding was provided by the New Zealand Foundation for Research, Science, and Technology (under C04X0302 to Forest Research) and the New Zealand Forest Health Collaborative.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gibb, A.R., Comeskey, D., Berndt, L. et al. Identification of Sex Pheromone Components of a New Zealand Geometrid Moth, the Common Forest Looper Pseudocoremia suavis, Reveals a Possible Species Complex. J Chem Ecol 32, 865–879 (2006). https://doi.org/10.1007/s10886-006-9031-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9031-1