Abstract
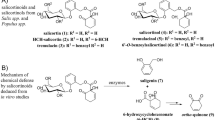
Within the genus Papilio, the P. glaucus group contains the most polyphagous Papilio species within the Papilionidae. The majority of Papilio species are associated with hostplants in the families Rutaceae and Apiaceae, and characterizing most are secondary metabolites called furanocoumarins. Recent phylogenetic studies suggest that furanocoumarin metabolism is an ancestral trait, with the glaucus group derived from ancestors associated with furanocoumarin-containing Rutaceae. In this study, we examined this relationship by conducting a gravimetric analysis of growth that used various concentrations of the furanocoumarin xanthotoxin. Papilio multicaudatus, the putative ancestor of the glaucus group, includes at least one furanocoumarin-containing rutaceous species among its hostplants; this species can consume leaf tissue containing up to 0.3% xanthotoxin with no detectable effect on relative growth rate, relative consumption rate, or efficiency of conversion of ingested food. As is the case for other Papilio species, xanthotoxin metabolism is mediated by cytochrome P450 monooxygenases (P450s). Ingestion of xanthotoxin by ultimate instar P. multicaudatus increases activity up to 30-fold in a dose-dependent fashion. Midguts of induced larvae can also effectively metabolize six other furanocoumarins, including both linear (bergapten, isopimpinellin, imperatorin) and angular (angelicin, sphondin) forms. A metabolite of xanthotoxin in the frass from xanthotoxin-treated larvae, identified as 6-(7-hydroxy-8-methoxycoumaryl)-acetic acid by MS–MS and NMR analyses, is identical to one from the frass of P. polyxenes. The occurrence of this metabolite in two swallowtails and the presence of a second metabolite of xanthotoxin, 6-(7-hydroxy-8-methoxycoumaryl)-hydroxyethanol in the frass of both P. polyxenes and Depressaria pastinacella are consistent with the suggestion that lepidopterans share as the first step of xanthotoxin metabolism the P450-mediated epoxidation of the furan ring 2′–3′ double bond.







Similar content being viewed by others
References
Aubert, J., Legal, L., Descimon, H., and Michel, F. 1999. Molecular phylogeny of swallowtail butterflies of the tribe Papilionini (Lepidoptera: Papilionidae). Mol. Phylogenet. Evol. 12:156–167.
Berenbaum, M. R. 1983. Coumarins and caterpillars: a case for coevolution. Evolution 37:163–179.
Berenbaum, M. R. 1995. Chemistry and oligophagy in the Papilionidae, pp. 27–38, in J. M. Scriber (ed.). Swallowtail Butterflies: Ecology and Evolutionary Biology. Scientific Publishers, Gainesville.
Berenbaum, M. R., Favret, C., and Schuler, M. A. 1996. On defining “key innovations” in an adaptive radiation: cytochrome P450s and Papilionidae. Am. Nat. 148:S139–A155.
Caterino, M. S. and Sperling, F. A. H. 1999. Papilio phylogeny based on mitochondrial cytochrome oxidase I and II genes. Mol. Phylogenet. Evol. 11:122–137.
Cohen, M. B., Berenbaum, M. R., and Schuler, M. A. 1989. Induction of cytchrome P450-mediated detoxification of xanthotoxin in the black swallowtail. J. Chem. Ecol. 15:2347–2354.
Cohen, M. B., Schuler, M. A., and Berenbaum, M. R. 1992. A host-inducible cytochrome P-450 from a host-specific caterpillar: molecular cloning and evolution. Proc. Natl. Acad. Sci. USA 89:10920–10924.
Dean, M. and Annilo, T., 2005. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics Hum. Genet. 6:123–142.
Ehrlich, P. R. and Raven, P. H. 1964. Butterflies and plants: a study in coevolution. Evolution 18:586–608.
Feeny, P. 1992. The evolution of chemical ecology: contributions from the study of herbivorous insects, pp. 1–44, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores Their Interactions with Secondary Plant Metabolites, Vol. 2. Academic Press, New York.
Hagen, R. H. and Scriber, J. M. 1991. Systematics of Papilio glaucus and P. troilus species groups (Lepidoptera: Papilionidae): inferences and allozymes. Ann. Entomol. Soc. Am. 84:380–395.
Hayes, J. D. and Pulford, D. J. 1995. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 30:445–460.
Ivie, G. W., Bull, D. L., Beier, R. C., Pryor, N. W., and Oertli, E. H. 1983. Metabolic detoxification: mechanism of insect resistance to plant psoralens. Science 221:374–376.
Li, W., Schuler, M. A., and Berenbaum, M. R. 2001. Molecular analysis of multiple CYP6B genes from polyphagous Papilio species. Insect Biochem. Mol. Biol. 31:999–1011.
Li, W., Petersen, R. A., Schuler, M. A., and Berenbaum, M. R. 2002. CYP6B cytochrome P450 monooxygenases from Papilio canadensis and Papilio glaucus: potential contributions of sequence divergence to host plant associations. Insect Mol. Biol. 11:543–551.
Li, W., Schuler, M. A., and Berenbaum, M. R. 2003. Diversification of furanocoumarin-metabolizing cytochrome P450 monooxygenases in two papilionids: Specificity and substrate encounter rate. Proc. Natl. Acad. Sci. USA 100:14593–14598.
Munroe, E. 1961. The classification of the Papilionidae (Lepidoptera). Can. Entomol., Suppl. 17:1–51.
Murray, R. D. H., Mendez, J., and Brown, S. A. 1982. The Natural Coumarins: Occurrence, Chemistry, and Biochemistry. John Wiley & Sons, Bristol, 702 pp.
Nitao, J. K., Berhow, M., Duval, S. M., Weisleder, D., Waughn, S. F., Zangerl, A., and Bernenbaum, M. R. 2003. Characterization of furanocoumarin metabolites in parsnip webworm, Depressaria pastinacella. J. Chem. Ecol. 29:671–682.
Raubenheimer, D. and Simpson, S. J. 1992. Analysis of covariance: an alternative to nutritional indices. Entomol. Exp. Appl. 62:221–231.
Reed, R. D. and Sperling, F. A. H. 1999. Interaction of process partitions in phylogenetic analysis: An example from the swallowtail butterfly genus Papilio. Mol. Biol. Evol. 16:286–297.
Schmid, J., Prox, A., Reuter, A., Zipp, H., and Koss, W. H. 1980. The metabolism of 8-methoxypsoralen in man. Eur. J. Drug Metab. Pharmacokinet. 5:81–92.
Scriber, J. M. 1995. Overview of swallowtail butterflies: taxonomic and distributional latitudes, pp. 3–8, in J. M. Scriber, Y. Tsubaki, and R. C. Lederhouse (eds.). Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scientific Publ., Gainesville.
Scriber, J. M., Lederhouse, R., and Hagen, R. 1991. Foodplant and evolution within P. glaucus and P. troilus species groups (Lepidoptera: Papilionidae), pp. 341–373, in P. W. Price, T. M. Lewinsohn, and W. W. Benson (eds.). Plant–Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. John Wiley & Sons, New York.
Sperling, F. A. H. 1993. Mitochondrial DNA variation and Haldane's rule in the Papilio glaucus and P. troilus species group. Heredity 71:227–233.
Sperling, F. A. H. and Harrison, R. G. 1994. Mitochondrial DNA variation within and between species of the Papilio machaon group of swallowtail butterflies. Evolution 48:408–422.
Vane-Wright, R. I., Raheem, D. C., Cieslak, A., and Vogler, A. P. 1999. Evolution of the mimetic African swallowtail butterfly Papilio dardanus: Molecular data confirm relationships with P. phorcas and P. constantinus. Biol. J. Linn. Soc. Lond. 66:215–229.
Waldbauer, G. P. 1968. The consumption and utilization of food by insects. Adv. Insect Physiol. 5:229–288.
Yagi, T., Sasaki, G., and Takebe, H. 1999. Phylogeny of Japanese papilionid butterflies inferred from nucleotide sequences of mitochondrial ND5 gene. J. Mol. Evol. 48:42–48.
Zakharov, E. V., Caterino, M. S., and Sperling, F. A. H. 2004. Molecular phylogeny, historical biogeography, and divergence time estimates for swallowtail butterflies of the genus Papilio (Lepidoptera: Papilionidae). Syst. Biol. 53:193–215.
Acknowledgments
The authors gratefully acknowledge the help of Dr. Furong Shun in MS–MS analysis of the metabolite. This work was supported by NSF IBN0212242 to MRB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mao, W., Berhow, M.A., Zangerl, A.R. et al. Cytochrome P450-Mediated Metabolism of Xanthotoxin by Papilio multicaudatus . J Chem Ecol 32, 523–536 (2006). https://doi.org/10.1007/s10886-005-9018-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-9018-3