Abstract
Changes have been made to the AnaConDa device (Sedana Medical, Stockholm, Sweden), decreasing its size to reduce dead space and carbon dioxide (CO2) retention. However, this also involves a decrease in the surface area of the activated carbon filter. The CO2 elimination and sevoflurane (SEV) reflection of the old device (ACD-100) were thus compared with the new version (ACD-50) in patients sedated after coronary artery bypass graft surgery. After ERC approval and written informed consent, 23 patients were sedated with SEV, using first the ACD-100 and then the ACD-50 for 60 min each. With each device, patients were ventilated with tidal volumes (TV) of 5 ml/kg of ideal body weight for the first 30 min, and with 7 ml/kg for the next 30 min. Ventilation parameters, arterial blood gases, Bispectral-Index™ (BIS, Aspect Medical Systems Inc., Newton, MA, USA), SEV concentrations exhaled by the patient (SEV-exhaled) and from the expiratory hose (SEV-lost) were recorded every 30 min. A SEV reflection index was calculated: SRI [%] = 100 × (1 − (SEV-lost/SEV-exhaled)). Data were compared using ANOVA with repeated measurements and Student’s T-tests for pairs. Respiratory rates, tidal and minute volumes were not significantly different between the two devices. End tidal and arterial CO2 partial pressures were significantly higher with the ACD-100 as compared with the ACD-50. SEV infusion rate remained constant. SEV reflection was higher (SRI: ACD-100 vs. ACD-50, TV 5 ml/kg: 95.29 ± 6.45 vs. 85.54 ± 11.15, p = 0.001; 7 ml/kg: 93.42 ± 6.55 vs. 88.77 ± 12.26, p = 0.003). BIS was significantly lower when using the higher TV (60.91 ± 9.99 vs. 66.57 ± 8.22, p = 0.012), although this difference was not clinically relevant. During postoperative sedation, the use of ACD-50 significantly reduced CO2 retention. SEV reflection was slightly reduced. However, patients remained sufficiently sedated without increasing SEV infusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sedation by inhalation occurs through the action of anaesthetics breathed in, used in the same manner as in anaesthesia during operations, although at a lower dose. These inhaled agents are minimally metabolized and thanks to their low solubility, are rapidly eliminated via the lungs. Because of these characteristics, such agents have been used as sedatives in an intensive care setting and have shown a shorter and more predictable time to awakening than occurs in sedation with intravenous agents [1].
Enlund et al. described the use of the reflection method in clinical practice for the first time in 2001. Reflection systems retain the anaesthetic during expiration and resupply it during the next inspiration. The AnaConDa® or Anaesthetic Conserving Device (Sedana Medical, Stockholm, Sweden) was the first medical product that permitted the efficient administration of volatile anaesthetic by the reflection technique [2]. Another device has been developed, the Mirus (Pall Medical, Dreieich, Germany), and it is the first to administer desflurane using reflection. The Mirus system can also be used for the delivery of sevoflurane and offers automatic target control of the end-tidal anaesthetic concentration [3].
The AnaConDa® can be used with any critical care ventilator and enables direct administration of the anaesthetic in liquid form by means of an infusion pump. Gas exhaled by the patient is absorbed by a filter made of activated carbon fibres interwoven into a felt, and it is then returned to the patient in the next inhalation.
Development of the AnaConDa® (ACD-100) device dates back to 2004. The instructions for use recommend a minimal tidal volume of 350 ml, with an internal volume of 100 ml. A new design for the device was introduced at the beginning of 2017, decreasing the internal volume to 50 ml, and thereby extending the scope of indications for its use. This reduction also involved a decrease in the size of the activated carbon filter.
As the AnaConDa’s functionality is based on the filter’s reflection efficiency, the aim of this study was to compare sevoflurane concentrations in the expiratory branch of the anaesthetic circuit. The concentration of sevoflurane exhaled by the patient was to be contrasted with the concentration on the ventilator side of the reflector. The proposal was therefore to compare the sevoflurane (SEV) reflection efficiency of the older device with that of the new device, known as AnaConDa-S® (ACD-50). The secondary objective was to assess the ventilation parameters and blood gas parameters obtained from the two devices.
2 Materials and methods
This was an analytical observational study with consecutively selected patients conducted in the recovery unit of a tertiary hospital from October 1, 2017 till December 15, 2017. The study was approved by the Clinical Research and Ethics Committee (Registration number: 17114/17, Leon Hospital, Leon, Spain). Written informed consent was obtained the day before surgery. Patients over 18 years of age undergoing scheduled coronary surgery with cardiopulmonary bypass (CPB) could be included. Exclusion criteria were as follows: body mass index (BMI) > 35 kg/m2, hypoxemic respiratory failure after CPB, history of moderate to severe pulmonary hypertension, history of chronic obstructive pulmonary disease, history of alcohol or psychotropic drug abuse, and history of malignant hyperthermia.
In order to compare reflection efficiency between the two devices, a SEV reflection index (SRI) was defined. It was known that the reflection efficiency of the AnaConDa® can be calculated in vitro using the formula: 100 × (1 − (SEV lost/SEV exhaled)) [4]. In estimating the sevoflurane lost and exhaled, gas was sampled at the AnaConDa® sampling port and at the end of the expiratory hose of the ICU ventilator with two gas monitors (Vamos®, Dräger Medical, Lübeck, Germany; Fig. 1). In this way, the SRI was estimated as equal to 100 × (1 − (SEV expiratory hose/SEV AnaConDa® sampling port)).
The variables recorded related to anthropometric data, such as weight, height and BMI. Height was used to determine the idealized weight in accordance with the formula (height in cm − 152.4) × 0.91, with 45.5 added to the result for women and 50 for men. Similarly, the sevoflurane and remifentanil infusion rates were recorded, corresponding to standard sedation analgesia. Using the established cut-offs and the arterial blood gas test, values were obtained for partial pressure of carbon dioxide (pCO2) and partial pressure of oxygen (PaO2) in blood. Evita XL® and Evita 2® ventilators (Dräger Medical, Lübeck, Germany), were utilized to obtain ventilation parameters. The sedation level was monitored through the bi-spectral index (BIS). In respect of haemodynamic variables, the mean arterial pressure (MAP) and the need for support with vasoactive drugs were noted.
Maintenance of anaesthesia in the operating room was ensured in all cases by using SEV and remifentanil. At the time of anaesthetic induction, a loading dose of 5 µg/kg of fentanyl was administered. All procedures were performed with cardiopulmonary bypass, with maintenance of SEV administration during this time. Transfer from the operating room to the ICU was achieved with the administration of boluses of 30 mg of propofol and fentanyl at a dose of 1 µg/kg.
Upon admission to the recovery unit, patients were monitored conventionally with an electrocardiograph, and by taking measurements of the invasive arterial pressure, oxygen saturation and depth of anaesthesia. The AnaConDa® devices were purged by using the bolus function of the syringe pump with the volumes recommended by the manufacturer. The ventilator’s parameters were set to volume control mode auto-flow, with an estimated tidal volume for the idealized weight of 5 ml/kg, with an I to E ratio of 1:2 and a respiratory rate for the end-tidal CO2 between 40 and 45 mmHg measured by capnography. Sedation was commenced with an ACD-100, with an initial arterial blood gas sample analysis and recording of variables 30 min after arrival. After the collection of these data, the ventilation parameters were changed to 7 ml/kg, in accordance with the aims mentioned above, and a new arterial blood gas test and recording of spirometry values was performed after 60 min. The respiratory circuit was then changed to an ACD-50, with the ventilator settings returned to 5 ml/kg for 30 min while data were gathered. After 90 min, the settings were changed to 7/ml/kg ready for the final data collection at 120 min. After this was done, the feasibility of extubation after 120 min of admission was assessed in accordance with the unit’s standard protocol. Data were collected manually by three investigators, with a precision of one decimal point in sevoflurane concentrations.
Sedation was maintained with SEV and remifentanil on the lines of this protocol. Any need for haemodynamic support with vasoactive drugs was determined by the attending physician, who was also responsible for modifying the sedation parameters, aiming for BIS levels between 60 and 80 and an end-tidal SEV concentrations of between 0.8 and 1.0 Vol%.
The sample size needed for the comparison of reflection efficiency was calculated to allow a comparison of means study incorporating related samples. It was assumed that the value retained with the ACD-100 device would be 90% and with the ACD-50 80%. Hence, for a power of 80% and a significance level of 5% at a standard deviation of 16% of the difference of the means, the size required was estimated at 26 patients. It was predicted that 3 patients would drop out of the study (10% loss rate).
The data were entered into a Microsoft Excel® database. For statistical analyses, use was made of the SPSS® 15.0 V2.0 program. In order to achieve the basic objective of comparing the SRI, Student’s T-test for paired data and an ANOVA with Bonferroni post hoc correction were employed. In respect of the secondary objectives of comparing pCO2 and ventilator parameters the same tests were used. For qualitative variables (the employment of vasoactive drugs) a Chi square test was utilized.
3 Results
The total sample consisted of 27 patients. Two patients were excluded because of histories of chronic obstructive pulmonary disease, one because postoperative sedation with propofol alone was felt to be optimal because of a suspected neurological event, and one was excluded owing to a history of alcoholism (> 60 g/day). Of the 23 patients included, 20 were men and 3 were women. Their mean weight was 75.42 kg (95% Confidence Interval 70.88–79.95), their mean height was 164.48 cm (95% CI 160.45–168.51), their mean BMI was 27.85 kg/m2 (95% CI 26.54–29.17), and their mean idealized weight was 60.61 kg (95% CI 56.52–64.70).
The comparative analyses were carried out for the pairs corresponding to minutes 30 and 90, and 60 and 120 min 30 with ACD-100 and a tidal volume of 5 ml/kg was compared to minute 90 with ACD-50 and the same ventilation parameters. Minute 60 with ACD-100 was compared to minute 120 with ACD-50, both having ventilation parameters set at 7 ml/kg.
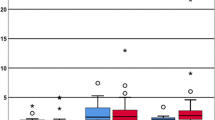
The data concerning the dosage of sedative and the sedation objectives measured with the BIS scale showed no statistically significant differences, except for the BIS at minutes 30 and 120. Here there was statistical significance but no clinical relevance, because both values lay within the objective clinical range of 60 to 80 (Table 1).
The blood gas analyses showed significant differences in pCO2, with lower values with the ACD-50, when the same tidal volumes were used. Capnography values with the Vamos monitor® showed significantly lower pCO2 values with the ACD-50 (Table 1).
With regard to haemodynamic details, the MAP at minutes 30 and 90 showed no significant differences (Table 1). An analysis comparing the proportions of use of vasoconstrictors, vasodilators and inotropic agents showed no statistically significant results (Table 1).
Respiratory rates and plateau pressure were not significantly different between devices. The ANOVA clearly shows differences in the values for expiratory tidal volumes when ventilation was at 5 ml/kg as against 7 ml/kg, but no differences were found among groups by means of Student’s T-test when ventilation was with the same volume (Table 1).
Significant differences were also found in the SEV concentrations exhaled by the patient between minutes 60 and 120 and in the expiratory hose of the circuit between minutes 30 and 90, and 30 and 120, with a lower concentration of SEV in the case of the ACD-100 ventilating at 5 ml/kg (Table 2).
With regard to the reflection efficiency, SRI of ACD-100 was significantly higher compared to ACD-50 when using 5 ml/kg tidal volume, but not when using 7 ml/kg, as assessed by Student’s T-test. Similarly, when comparing all four time points using ANOVA, we found a significant difference between ACD-50 at 5 ml/kg tidal volume compared to ACD-100 independent of the tidal volume, with an SRI significantly lower in the case of the ACD-50 (Table 2).
4 Discussion
The reflection efficiency of the activated carbon filter of the ACD-50 under standard practice conditions was lower than that of the ACD-100, especially when ventilating with volumes of 5 ml/kg of idealized weight. The ACD-100 activated carbon filter is theoretically capable of absorbing 90% of the patient’s exhaled gas [5]. With established infusion models, infusion rates of 10 ml/h are rarely exceeded in clinical practice [6], which implies that the filter size might be excessive. It was thus surmised that the size of the device could be reduced without affecting its performance, thereby improving its operating limitations. These limitations are determined by the need for a minimum tidal volume of 350 ml, because the ACD-100 requires the insertion of an internal volume of 100 ml [7, 8] into the circuit. This restricts its use with paediatric populations and also for acute respiratory distress syndrome with protective ventilation parameters. On the basis of these premises, a “new” AnaConDa was designed, with an internal volume of 50 ml and minimum tidal volume of 200 ml [9], permitting a significant reduction in the size of the activated carbon filter, while preserving its operational characteristics.
The internal volume of the reflection system determines the volumetric dead space. In addition, some carbon dioxide is reflected back to the patient. The tidal volume increase necessary to maintain normal arterial carbon dioxide pressure, or normocapnia, has been termed reflective dead space [10]. Reflective dead space has been quantified as 40 ml for the ACD-100, and 25 ml for ACD-50 [4]. Hence, the total device dead space, that is, the sum of volumetric and reflective dead space, is 140 ml for the ACD-100 and 75 ml for the ACD-50.
In order to assess reflection efficiency, a new index was defined, named the Sevoflurane Reflection Index (SRI). The aim was to develop a “clinical” index of the reflection capacity of the AnaConDa® that avoided assumptions made in other theoretical models that use artificial lungs [5, 11]. Such models estimate lost SEV and SEV in the patient in a balanced phase, in other words under steady state conditions. In this way, administration of SEV in the system is equal to the lost SEV exiting through the reflector. Thus, the concept of reflection efficiency is based on the amount of SEV exhaled or lost or re-inspired in the sense of net volume of gas. In this instance, concentrations of gas are measured, as it is not possible to calculate with precision the amount of SEV that is lost, since this is not a balanced phase. Even so, the SRI estimates the reflection capacity under real conditions in a simple manner that is clinically reproducible. Hence, it can be used for making comparisons between various types of apparatus and different volatile anaesthetics.
In this study, it was observed that with the classical device the reflection index for the anaesthetic gas exceeded 90%, with tidal volumes between 5 and 7 ml/kg, while with the new device the equivalent values were below 90% with no differences in tidal volumes. There is an inconsistent finding in the data, in that the SRI was less with 5 ml/kg than with 7 ml/kg, in view of the fact that the filter’s in vitro reflection efficiency is supposed to be inversely related to the product of the gas concentration and programmed tidal volume [11]. On the basis of the data analysed, a possible explanation for this finding is that use in standard practice involves changes in temperature and humidity which may affect the filter’s reflection efficiency [12].
It is important to note that despite the decrease in reflection efficiency, infusion rates of sevoflurane showed no differences between the ACD-100 and the ACD-50. Similarly, the values for the bi-spectral index were in the same range, with statistical but no clinical differences.
The environmental pollution implications of this lower reflection efficiency are still to be assessed. However, it is known that with the previous characteristics the environmental pollution limits recommended by the United States National Institute for Occupational Safety and Health of two parts per million were not exceeded [13]. There were no changes in the handling of the device or the loading of the syringe [14], so the risks are currently the same for both.
The data showed no differences in the minute volumes obtained with the two devices, although the arterial pCO2 and Vamos© monitor pCO2 values were lower with the ACD-50. This might be because of lower CO2 retention in this device which has a smaller device dead space. Reflective dead space was attenuated by volatile agents [9, 15], but these effects of sevoflurane do not seem sufficient to compensate for the decrease in the total device space in the ACD-50. This pCO2 reduction does not seem to correspond to haemodynamic impairment, as the MAP showed no differences between the groups, although this is not truly an appropriate measure of cardiac output. There were no differences in the oxygenation values between the devices. In terms of the respiratory mechanics study, the devices did not affect plateau pressures. Previous studies have assessed the influence of the ACD-100 on respiratory effort [16] and concluded that introducing a gas (sevoflurane) into the circuit minimizes the influence of the device, with characteristics approximating to those of the humidifier filters commonly employed.
The data analysed showed a slightly lower reflection efficiency in the ACD-50, but sevoflurane infusion rates were not increased, since an adequate depth of sedation was maintained. These data lead to the question of what real clinical relevance this decrease in reflection efficiency might have. On the other hand, there was a significantly lower pCO2, with the AnaConDa-S®, differences being close to 5 mmHg. This might by of clinical significance, for example in patients with respiratory distress syndrome or intracranial hypertension.
In the same line, Bomberg and cols. recently published a paper with 10 patients sedated with isoflurane in spontaneous breathing comparing ACD-100 versus ACD-50 during 5 h observation period. They found that ACD-50 reduces tidal volumes and end-tidal carbon dioxide concentrations. Respiratory rate, hemodynamic variables, sedation depth and isoflurane infusion rates remained unchanged [17]. These results are concordant with ours, but in this case with patients in spontaneous breathing.
The setting for implementing this study was cardiac surgery. In this context of cardiac patients sedation with halogenated compounds is especially indicated [18], as it decreases the incidence of pulmonary complications [19]. Measurements were performed under standard practice conditions so as to determine the applicability of the new apparatus in such a context, in an effort to help clinicians understand this device. To improve homogeneity of the sample obesity, history of chronic obstructive pulmonary disease, history of alcohol or psychotropic drug abuse were included as exclusion criteria, although volatile anaesthetics seems useful in these populations [20].
The limitations of this study were determined by this standard practice. The patients were ventilated in a manner that allowed for interaction with the patient, in that if the patient did not present neuromuscular relaxation, the spirometry values were not only programmed by the clinician but also showed this interaction with the patient. This might affect the tidal volume and minute volume data. It would have been advisable to use a single ventilator to collect data, but this choice would have interfered with the regular work-flow of the recovery unit and the study would have ceased to have strictly observational value. On these same lines, the Vamos® monitor displays the sevoflurane concentration only to one decimal place; manual recording of this might affect the accuracy of measurements.
Future lines of study and aspects to be considered with regard to this new device include the influence of its size on respiratory effort and an assessment of dead space using volumetric capnography measurements. In terms of pollution, future studies should assess whether this reduction in retention capacity has any effect of contaminating the environment.
In conclusion, reducing the size of the AnaConDa® and hence that of its activated carbon filter affected the conditions of standard practice in terms of sevoflurane reflection efficiency. With the ACD-100, the efficiency index exceeded 90%. The ACD-50, however, showed a slightly lower efficiency, with programmed tidal volumes of 5 ml and 7 ml per kg of idealized weight. Despite the lower reflection efficiency of the ACD-50, infusion rates for sevoflurane and BIS values showed no difference between the two versions of the apparatus. A significant reduction in arterial pCO2 was observed with the ACD-50 because of the lower total device dead space.
Outline design of the study. Sevoflurane measurements were performed using Vamos® monitors (Dräger, Germany) in the gas sampling port and expiratory branch of the AnaConDa (Sedana Medical, Sweden), next to the ventilator connection. With the same patient, blood gas measurements were performed at minutes 30, 60, 90 and 120 during the postoperative period, the first two with the “older” device and the last two with the “new” device
References
Spencer EM, Willatts SM. Isoflurane for prolonged sedation in the intensive care unit; efficacy and safety. Intensive Care Med. 1992;18:415–21.
Bomberg H, Volk T, Groesdonk HV, Meiser A. Efficient application of volatile anaesthetics: total rebreathing or specific reflection? J Clin Monit Comput. 2018;32:615–22.
Bomberg H, Glas M, Groesdonk VH, Bellgardt M, Schwarz J, Volk T, Meiser A. A novel device for target controlled administration and reflection of desflurane—the mirus. Anaesthesia. 2014;69:1241–50.
Bomberg H, Meiser F, Daume P, Bellgardt M, Volk T, Sessler DI, Groesdonk HV, Meiser A. Halving the volume of AnaConDa: evaluation of a new small-volume anesthetic reflector in a test lung model. Anesth Analg. 2018. (Electronic publication prior to print version).
Meiser A, Bellgardt M, Belda J, Röhm K, Laubenthal H, Sirtl C. Technical performance and reflection capacity of the anesthetic conserving device—a bench study with isoflurane and sevoflurane. J Clin Monit Comput. 2009;23:11–9.
Belda JF, Soro M, Badenes R, Meiser A, García ML, Aguilar G, Martí FJ. The predictive performance of a pharmacokinetic model for manually adjusted infusion of liquid sevoflurane for use with the anaesthetic-conserving device (AnaConDa). A clinical study. Anesth Analg. 2008;106:1207–14.
Sturesson LW, Bodelsson M, Johansson A, Jonson B, Malmkvist G. Apparent dead space with the anesthetic conserving device, AnaConDa©: a clinical laboratory investigation. Anesth Analg. 2013;117:1319–24.
Sturesson LW, Malmkvist G, Bodelsson M, Niklason L, Jonson B. Carbon dioxide rebreathing with the anaesthetic conserving device, AnaConDa©. Br J Anaesth. 2012;109:279–83.
Farrell R, Oomen G, Carey P. A technical review of the history, development and performance of the anaesthetic conserving device “AnaConDa” for delivering volatile anaesthetic in intensive and post-operative critical care. J Clin Monit Comput. 2018;32:595–604.
Bomberg H, Veddeler M, Volk T, Groesdonk HV, Meiser A. Volumetric and reflective dead space of anaesthetic reflectors under different conditions. J Clin Monit Comput. 2018;32:1073–80. (Electronic publication prior to print version).
Bomberg H, Wessendorf M, Bellgardt M, Veddeler M, Wagenpfeil S, Volk T, Groesdonk HV, Meiser A. Evaluating the efficiency of desflurane reflection in two commercially available reflectors. J Clin Monit Comput. 2016;32:605–14 (Electronic publication prior to print version).
Sturesson LW, Frennstrom JO, Ilardi M, Reinstrup P. Comparing charcoal and zeolite reflection filters for volatile anaesthetics. Eur J Anaesthesiol. 2015;32:521–6.
Pickworth T, Jerath A, DeVine R, Kherani N, Wasowics M. The scavenging of volatile anesthetic agents in the cardiovascular intensive care unit environment: a technical report. Can J Anesth. 2013;60:38–43.
Soukup J, Scharff K, Kubosch K, Pohl C, Bompltz M, Kompardt J. State of the art: sedation concepts with volatile anesthetics in critically Ill patients. J Crit Care. 2009;24:535–44.
Sturesson LW, Bodelsson M, Jonson B, Malmkvist G. Anaesthetic conserving device AnaConDa®: dead space effect and significance for lung protective ventilation. Br J Anaesth. 2014;113:508–14.
Chabanne R, Perbet S, Futier E, Ben Said NA, Jaber S, Bazin JE, Pereira B, Constantin JM. Impact of the anesthetic conserving device on respiratory parameters and work of breathing in critically Ill patients under light sedation with sevoflurane. Anesthesiology. 2014;121:808–16.
Bomberg H, Meiser F, Zimmer S, Bellgardt M, Volk T, Sessler DI, Groesdonk HV, Meiser A. Halving the volume of AnaConDa: initial clinical experience with a new small-volume anaesthetic reflector in critical ill patients—a quality improvement project. J Clin Monit Comput. 2018;32:639–46.
Liu H, Ji F, Peng K, Applegate RL, Fleming N. Sedation after cardiac surgery: is one drug better than another? Anesth Analg. 2017;124:1061–70.
Uhlig C, Bluth T, Schwarz K, Deckert S, Heinrich L, De Hert S, Landoni G, Serpa Neto A, Schultz MJ, Pelosi P, Schmitt J, Gama de Abreu M. Effects of volatile anesthetics on mortality and postoperative pulmonary and other complications in patients undergoing surgery: a systematic review and meta-analysis. Anesthesiology. 2016;124:1230–45.
Ruszkai Z, Bokrétás GP, Bartha PT. Sevoflurane therapy for life-threatening acute severe asthma: a case report. Can J Anaesthesiol. 2014;61:943–50.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were inaccordance with the ethical standards of the Institutional and Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marcos-Vidal, J.M., Merino, M., González, R. et al. Comparison of the use of AnaConDa® versus AnaConDa-S® during the post-operative period of cardiac surgery under standard conditions of practice. J Clin Monit Comput 34, 89–95 (2020). https://doi.org/10.1007/s10877-019-00285-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-019-00285-0