Abstract
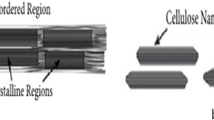
Cellulose nanocrystals (CNC) obtained from sawdust were modified with chitosan (CHT), as green coagulant (CNC/CHT) for the removal of Erichrome black-T (EBT) dye from aqueous solution. The pristine CNC, CNC/CHT and CNC/CHT-EBT floccules formed after the coagulation process were characterized by different techniques. The diffraction pattern of CNC/CHT showed peaks for both CNC and CHT, therefore, confirmed the co-regeneration of cellulose–chitosan mixing. In addition, the band at 1586 cm−1 found in the infra-red spectrum of this composite, which was attributed to the NH bending of primary amine, and a major functional group of the chitosan, was an indication of the incorporation of chitosan with the CNC. Three different ratios of the CNC:CHT were explored in order to determine the best modification regime for EBT coagulation. Different parameters, including solution pH, coagulant dosage, settling time, initial dye concentration and effect of material ratios were studied. The maximum coagulation of 99.9% was achieved at the optimum pH value of 2.10 using 100 mg/L of EBT concentration. Overall, the performance achieved using the green synthesized CNC/CHT, within the maximum settling time of 30 min, confirmed the efficiency and cost effectiveness of this coagulant for the removal of EBT from water.










Similar content being viewed by others
References
M. A. Aly-Eldeen, et al. (2018). The uptake of Eriochrome Black T dye from aqueous solutions utilizing waste activated sludge: adsorption process optimization using factorial design. Egypt. J. Aquat. Res. 44 (3), 179–186.
F. Moeinpour, A. Alimoradi, and M. Kazemi (2014). Efficient removal of Eriochrome black-T from aqueous solution using NiFe2O4 magnetic nanoparticles. J. Environ. Health Sci. Eng. 12 (1), 112–112.
T. S. Kazeem, et al. (2020). Enhanced removal of Eriochrome Black T using graphene/NiMgAl-layered hydroxides: isotherm, kinetic, and thermodynamic studies. Arab. J. Sci. Eng. 45 (9), 7175–7189.
A. Mittal and V. K. Gupta (2010). Adsorptive removal and recovery of the azo dye Eriochrome Black T. Toxicol. Environ. Chem. 92 (10), 1813–1823.
P. Franco, et al. (2020). Photocatalytic degradation of Eriochrome Black-T azo dye using Eu-doped ZnO prepared by supercritical antisolvent precipitation route: a preliminary investigation. Top. Catal. 63 (11), 1193–1205.
S. Kansal, et al. (2013). Photocatalytic degradation of Eriochrome Black T dye using well-crystalline anatase TiO2 nanoparticles. J. Alloys Compds. 581, 392–397.
D. T. Cestarolli, A. das Graças de Oliveira, and E. M. Guerra (2019). Removal of Eriochrome Black textile dye from aqueous solution by combined electrocoagulation–electroflotation methodology. Appl. Water Sci. 9 (4), 101.
D. Cestarolli, A. Oliveira, and E. Guerra (2019). Removal of Eriochrome Black textile dye from aqueous solution by combined electrocoagulation–electroflotation methodology. Appl Water Sci. https://doi.org/10.1007/s13201-019-0985-x.
P. Cañizares, et al. (2006). Electrochemically assisted coagulation of wastes polluted with Eriochrome Black T. Ind. Eng. Chem. Res. 45 (10), 3474–3480.
H. Ahmad, et al. (2016). Comparison of coagulation, electrocoagulation and biological techniques for the municipal wastewater treatment. Int. J. Appl. Eng. Res. 11, 11014–11024.
A. Nkalane, et al. (2019). Application of coagulant obtained through charge reversal of sawdust-derived cellulose nanocrystals in the enhancement of water turbidity removal. Mater. Res. Express 6 (10), 105060.
O. A. Oyewo, et al. (2019). Highly efficient removal of nickel and cadmium from water using sawdust-derived cellulose nanocrystals. J. Environ. Chem. Eng. 7 (4), 103251.
Y.-K. Twu, et al. (2003). Preparation and sorption activity of chitosan/cellulose blend beads. Carbohydr. Polym. 54 (4), 425–430.
S. Olivera, et al. (2016). Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: a review. Carbohydr. Polym. 153, 600–618.
M. Gibril, et al. (2018). Optimisation and enhancement of crystalline nanocellulose production by ultrasonic pretreatment of dissolving wood pulp fibres. Cellul. Chem. Technol. 52, 9–10.
O. A. Oyewo, et al. (2020). Sawdust-based cellulose nanocrystals incorporated with ZnO nanoparticles as efficient adsorption media in the removal of methylene blue dye. ACS Omega 5 (30), 18798–18807.
O. A. Oyewo, et al. (2021). Adsorptive and coagulative removal of trace metals from water using surface modified sawdust-based cellulose nanocrystals. J 4 (2), 193.
J. P. de Mesquita, et al. (2012). Bio-based nanocomposites obtained through covalent linkage between chitosan and cellulose nanocrystals. Carbohydr. Polym. 90 (1), 210–217.
C. Z. Thou, et al. (2021). Surface charge on chitosan/cellulose nanowhiskers composite via functionalized and untreated carbon nanotube. Arab. J. Chem. 14 (3), 103022.
A. Khan, et al. (2012). Mechanical and barrier properties of nanocrystalline cellulose reinfroced chitosan based nanocomposites film. Carbohydr. Polym. 90, 1061–1068.
M. Szymańska-Chargot, et al. (2019). Influence of chitosan addition on the mechanical and antibacterial properties of carrot cellulose nanofibre film. Cellulose 26 (18), 9613–9629.
U. Sampath, et al. (2017). Preparation and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel. Cellulose 24, 2215.
Q. Xu, et al. (2019). Fabrication of cellulose nanocrystal/chitosan hydrogel for controlled drug release. Nanomaterials (Basel) 9 (2), 253.
D. Morgado, et al. (2011). Biobased films prepared from NaOH/thiourea aqueous solution of chitosan and linter cellulose. Cellulose 18, 699–712.
Y. Jia, et al. (2017). Preparation and characterization of a novel bacterial cellulose/chitosan bio-hydrogel. Nanomater. Nanotechnol. 7, 1847980417707172.
R. E. Abou-Zeid, et al. (2015). Use of cellulose and oxidized cellulose nanocrystals from olive stones in chitosan bionanocomposites. J. Nanomater. 16, 172.
V. Rubentheren, et al. (2015). Physical and chemical reinforcement of chitosan film using nanocrystalline cellulose and tannic acid. Cellulose 22, 2529.
K. Ssekatawa, et al. (2021). Isolation and characterization of chitosan from Ugandan edible mushrooms, Nile perch scales and banana weevils for biomedical applications. Sci. Rep. 11 (1), 4116.
P. A. Vinodhini, et al. (2017). FTIR, XRD and DSC studies of nanochitosan, cellulose acetate and polyethylene glycol blend ultrafiltration membranes. Int. J. Biol. Macromol. 104 (Pt B), 1721–1729.
M. K. Kamal, et al. (2018). Synthesis and optimization of novel chitosan/cellulose acetate natural polymer membrane for water treatment. J. Adv. Phys. 14, 5303–5311.
H. Celebi and A. J. C. P. Kurt (2015). Effects of processing on the properties of chitosan/cellulose nanocrystal films. Carbohydr. Polym. 133, 284–293.
A. Brinkmann, et al. (2016). Correlating cellulose nanocrystal particle size and surface area. Langmuir 32 (24), 6105–6114.
H. Liang, K. Liu, and Y. J. M. L. Ni (2015). Synthesis of mesoporous α-Fe2O3 via sol–gel methods using cellulose nano-crystals (CNC) as template and its photo-catalytic properties. Mater. Lett. 159, 218–220.
T. Zhang, et al. (2018). Characterization of the nano-cellulose aerogel from mixing CNF and CNC with different ratio. Mater. Lett. 229, 103–106.
J. Bu, et al. (2019). Enhanced removal of Eriochrome Black T in wastewater by zirconium-based MOF/graphene oxide. Can. J. Chem. 98 (2), 90–97.
H. Balouchi, et al. (2020). Combination of electrocoagulation and MOF adsorption systems for EBT removal from water. Int. J. Environ. Anal. Chem. 2020, 1–11.
J. Yang, et al. (2019). pH-responsive cellulose–chitosan nanocomposite films with slow release of chitosan. Cellulose 26, 3763.
J. Odiyo, et al. (2017). Coagulation efficiency of Dicerocaryum eriocarpum (DE) plant %. Water SA 43, 1–6.
N. Precious Sibiya, S. Rathilal, and E. K. Tetteh (2021). Coagulation treatment of wastewater: kinetics and natural coagulant evaluation. Molecules (Basel, Switzerland) 26 (3), 698.
K. N. M. Bernard, et al. (2019). Coagulation and sedimentation of concentrated laterite suspensions: comparison of hydrolyzing salts in presence of Grewia spp biopolymer. J. Chem. 2019, 1431694.
Y. Wei, et al. (2015). Characterisation and coagulation performance of an inorganic coagulant: poly-magnesium-silicate-chloride in treatment of simulated dyeing wastewater. Colloids Surf. 470, 137–141.
Ö. B. Gökçek and S. Özdemir (2020). Optimization of the coagulation-flocculation process for slaughterhouse wastewater using response surface methodology. Clean Soil Air Water 48 (7–8), 2000033.
O. Amuda and A. J. D. Alade (2006). Coagulation/flocculation process in the treatment of abattoir wastewater. Desalination 196 (1–3), 22–31.
Acknowledgements
The authors would like to acknowledge the University of Johannesburg under the Global Excellence Stature Fellowship (GES) and North-West University, South Africa for financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oyewo, O.A., Ramaila, S., Mavuru, L. et al. Chitosan Modified Sawdust-Derived Cellulose Nanocrystals as Green Coagulant for Erichrome Black T. J Clust Sci 34, 427–436 (2023). https://doi.org/10.1007/s10876-022-02227-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02227-4