Abstract
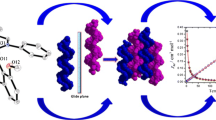
Schiff base ligands with multiple chelating coordination sites are usually able to quickly capture lanthanide metal ions to form complexes, and it is difficult to twist and construct helical chains. In this work, we used 2-hydroxy-1-naphthaldehyde, propylenediamine, and Ln(NO3)3·6H2O to react at 80 °C in solvothermal to obtain chains [Ln = Dy (1); Ln = Gd (2)] with a helical structure. It is worth noting that 2-hydroxy-1-naphthaldehyde and propylenediamine undergo an in situ reaction under one-pot conditions to form ligand 1,1′-((1E,1′E)-(propane-1,3-diylbis(azaneylylidene))bis(methaneylylidene))bis(naphthalen-2-ol) (H2L). The ligand (L)2− bridges the two metal center Ln(III) ions with a monodentate, causing it to twist into an “S” shape and further form a helical chain. In addition, the independent unit of the helical chain is a single core, and its metal center is in the O9 coordination environment provided by the ligand and the end group coordination NO3− ions. What’s more noteworthy is that chain 1 exhibits single-molecule magnet (SMM) behavior, and its SMM performance is significantly improved under the DC field induction condition of 600 Oe. By Debye model fitting, the effective energy barrier of 1 is 17.8 K, and the relaxation time is 2.4 × 10–6 s. The magnetocaloric effect test of chain 2 is further carried out. When ΔH = 7 T and T = 2 K, its − ΔSm reaches the maximum value of 16.19 J K−1 kg−1.
Graphic Abstract






Similar content being viewed by others
References
J.-L. Liu, Y.-C. Chen, F.-S. Guo, and M.-L. Tong (2014). Recent advances in the design of magnetic molecules for use as cryogenic magnetic coolants. Coord. Chem. Rev. 281, 26–49.
J.-H. Jia, Q.-W. Li, Y.-C. Chen, J.-L. Liu, and M.-L. Tong (2019). Luminescent single-molecule magnets based on lanthanides: design strategies, recent advances and magneto-luminescent studies. Coord. Chem. Rev. 378, 365–381.
P. Zhang, Y.-N. Guo, and J. Tang (2013). Recent advances in dysprosium-based single molecule magnets: structural overview and synthetic strategies. Coord. Chem. Rev. 257, 1728–1763.
Z.-H. Zhu, J.-M. Peng, H.-L. Wang, H.-H. Zou, and F.-P. Liang (2020). Assembly mechanism and heavy metal ion sensing of cage-shaped lanthanide nanoclusters. Cell Rep. Phys. Sci. 1 (8), 100165.
X.-Y. Li, H.-F. Su, Q.-W. Li, R. Feng, H.-Y. Bai, H.-Y. Chen, J. Xu, and X.-H. Bu (2019). A giant Dy76 cluster: a new fused Bi-nanopillar structural model in lanthanide clusters. Angew. Chem. Int. Ed. 58, 10184–10188.
L. Qin, Y.-Z. Yu, P.-Q. Liao, W. Xue, Z. Zheng, X.-M. Chen, and Y.-Z. Zheng (2016). A “Molecular Water Pipe”: a giant tubular cluster Dy72 exhibits fast proton transport and slow magnetic relaxation. Adv. Mater. 28, 10772–10779.
X.-J. Kong, Y. Wu, L.-S. Long, L.-S. Zheng, and Z. Zheng (2009). A chiral 60-metal sodalite cage featuring 24 vertex-sharing [Er4(μ3-OH)4] cubanes. J. Am. Chem. Soc. 131, 6918–6919.
X.-M. Luo, Z.-B. Hu, Q. Lin, W. Cheng, J.-P. Cao, C.-H. Cui, H. Mei, Y. Song, and Y. Xu (2018). Exploring the performance improvement of magnetocaloric effect based Gd-exclusive cluster Gd60. J. Am. Chem. Soc. 140, 11219–11222.
M. Wu, F. Jiang, D. Yuan, J. Pang, J. Qian, S. A. AL-Thabaiti, and M. Hong (2014). Polymeric double-anion templated Er48 nanotubes. Chem. Commun. 50, 1113–1115.
Z.-R. Luo, H.-L. Wang, Z.-H. Zhu, T. Liu, X.-F. Ma, H.-F. Wang, H.-H. Zou, and F.-P. Liang (2020). Assembly of Dy60 and Dy30 cage-shaped nanoclusters. Commun. Chem. 3 (1), 30. https://doi.org/10.1038/s42004-020-0276-3.
J. Dong, P. Cui, P.-F. Shi, P. Cheng, and B. Zhao (2015). Ultrastrong alkali-resisting lanthanide-zeolites assembled by [Ln60] nanocages. J. Am. Chem. Soc. 137, 15988–15991.
Y.-J. Ma, J.-X. Hu, S.-D. Han, J. Pan, J.-H. Li, and G.-M. Wang (2020). Manipulating on/off single-molecule magnet behavior in a Dy(III)-based photochromic complex. J. Am. Chem. Soc. 142, 2682–2689.
H.-L. Wang, J.-M. Peng, Z.-H. Zhu, K.-Q. Mo, X.-F. Ma, B. Li, H.-H. Zou, and F.-P. Liang (2019). Step-by-step and competitive assembly of two Dy(III) single-molecule magnets with their performance tuned by Schiff base ligands. Cryst. Growth Des. 19, 5369–5375.
K.-Q. Mo, Z.-H. Zhu, H.-L. Wang, X.-F. Ma, J.-M. Peng, H.-H. Zou, and F.-P. Liang (2019). Substituents lead to differences in the formation of two different butterfly-shaped NiII2DyIII2 clusters: structures and multistep assembly mechanisms. Dalton Trans. 48 (44), 16641–16649.
H.-L. Wang, T. Liu, Z.-H. Zhu, J.-M. Peng, H.-H. Zou, and F.-P. Liang (2021). A series of dysprosium clusters assembled by a substitution effect-driven out-to-in growth mechanism. Inorg. Chem. Front. 8, 2136–2143.
Z.-H. Zhu, X.-F. Ma, H.-L. Wang, H.-H. Zou, K.-Q. Mo, Y.-Q. Zhang, Q.-Z. Yang, B. Li, and F.-P. Liang (2018). A triangular Dy3 single-molecule toroic with high inversion energy barrier: magnetic properties and multiple-step assembly mechanism. Inorg. Chem. Front. 5, 3155–3162.
H.-L. Wang, X.-F. Ma, J.-M. Peng, Z.-H. Zhu, B. Li, H.-H. Zou, and F.-P. Liang (2019). Tracking the stepwise formation of the dysprosium cluster (Dy10) with multiple relaxation behavior. Inorg. Chem. 58, 9169–9174.
X.-F. Ma, H.-L. Wang, Z.-H. Zhu, B. Li, K.-Q. Mo, H.-H. Zou, and F.-P. Liang (2019). Formation of nanocluster Dy12 containing Dy-exclusive vertex-sharing [Dy4(μ3-OH)4] cubanes via simultaneous multitemplate guided and step-by-step assembly. Dalton Trans. 48, 11338–11344.
K.-Q. Mo, X.-F. Ma, H.-L. Wang, Z.-H. Zhu, Y.-C. Liu, H.-H. Zou, and F.-P. Liang (2019). Tracking the multistep formation of Ln(III) complexes with in situ Schif base exchange reaction and its highly selective sensing of dichloromethane. Sci. Rep. 9, 12231–12237.
A. Neshat, M. Gholinejad, H. Özcan, et al. (2021). Suzuki coupling reactions catalyzed by Schiff base supported palladium complexes bearing the vitamin B6 cofactor. Mol. Catal. 505, 111528.
N. C. Jana, P. Brandão, A. Frontera, et al. (2020). A facile biomimetic catalytic activity through hydrogen atom abstraction by the secondary coordination sphere in manganese(III) complexes. Dalton Trans. 49, 14216–14230.
Q. Zhang, J. Shu, Y. Zhang, et al. (2020). Structures and esterolytic reactivity of novel binuclear copper(II) complexes with reduced l-serine Schiff bases as mimic carboxylesterases. Dalton Trans. 49, 10261–10269.
F. Faridbod, M. R. Ganjali, R. Dinarvand, et al. (2008). Schiff’s bases and crown ethers as supramolecular sensing materials in the construction of potentiometric membrane sensors. Sensors. 8, 1645–1703.
A. J. Mayans, M. Bardia, B. L. Di, et al. (2018). Chiral [MnIIMnIII3M’] (M’ = NaI, CaII, MnII) and [MnIIMnIII6NaI2] clusters built from an enantiomerically pure Schiff base: synthetic, chiroptical and magnetic properties. Chem. Eur. J. 24, 18705–18717.
D. C. Liu, D. C. Zhong, and T. B. Lu (2020). Non-noble metal-based molecular complexes for CO2 reduction: from the ligand design perspective. EnergyChem. 2, 100034.
K. Zhang, V. Montigaud, O. Cador, G. P. Li, B. L. Guennic, J. Tang, and Y. Y. Wang (2018). Tuning the magnetic interactions in Dy(III)4 single-molecule magnets. Inorg. Chem. 57, 8550–8557.
G. Karotsis, S. Kennedy, S. J. Teat, C. M. Beavers, D. A. Fowler, J. J. Morales, M. Evangelisti, S. J. Dalgarno, and E. K. Brechin (2010). [MnIII4LnIII4] Calix[4]arene clusters as enhanced magnetic coolers and molecular magnets. J. Am. Chem. Soc. 132, 12983–12990.
J.-B. Peng, Q.-C. Zhang, X.-J. Kong, Y.-Z. Zheng, Y.-P. Ren, L.-S. Long, R.-B. Huang, L.-S. Zheng, and Z. Zheng (2012). High-nuclearity 3d–4f clusters as enhanced magnetic coolers and molecular magnets. J. Am. Chem. Soc. 134, 3314–3317.
C.-M. Liu, D.-Q. Zhang, X. Hao, and D.-B. Zhu (2020). Assembly of chiral 3d–4f wheel-like cluster complexes with achiral ligands: single-molecule magnetic behavior and magnetocaloric effect. Inorg. Chem. Front. 7, 3340–3351.
S.-D. Han, X.-H. Miao, S.-J. Liu, and X.-H. Bu (2014). Magnetocaloric effect and slow magnetic relaxation in two dense (3,12)-connected lanthanide complexes. Inorg. Chem. Front. 1, 549–552.
M.-L. Han, Y.-P. Duan, D.-S. Li, G.-W. Xu, Y.-P. Wu, and J. Zhao (2014). A series of divalent metal coordination polymers based on isomeric tetracarboxylic acids: synthesis, structures, and magnetic properties. Dalton Trans. 43, 17519–17527.
M.-L. Han, X.-H. Chang, X. Feng, L.-F. Ma, and L.-Y. Wang (2014). Temperature and pH driven self-assembly of Zn(II) coordination polymers: crystal structures, supramolecular isomerism, and photoluminescence. CrystEngComm 16, 1687–1695.
Z.-H. Zhou, M.-L. Han, Y.-P. Wu, W.-W. Dong, D.-S. Li, and J. Y. Lu (2016). N-donor co-ligands driven two new Co(II)- coordination polymers with bi- and trinuclear units: crystal structures, and magnetic properties. J. Solid State Chem. 242, 207–211.
M.-L. Han, X.-Q. Wu, G.-W. Xu, G.-X. Wen, D.-S. Li, and L.-F. Ma (2016). A Ni(II) ferromagnet with mixed pyridine-3,5-dicarboxylate-1,4-bis (imidazol-l-yl) butane heterobridges exhibiting long-range ordering and hysteresis loop. Inorg. Chem. Commun. 69, 31–34.
Y. A. Tyula, A. Zabardasti, H. Goudarziafshar, et al. (2017). Template synthesis of two new supramolecular zinc(II) complexes containing pentadentate N3O2 semicarbazone ligand: nanostructure synthesis, Hirshfeld surface analysis, and DFT studies. J. Mol. Struct. 1150, 383–394.
M. A. AlDamen, N. Charef, H. K. Juwhari, et al. (2016). Crystal structures, optical properties, and TD-DFT study of a zinc(II) Schiff-base complex derived from salicylaldehyde and N-1-(3-aminopropyl)-propane-1,3-diamine. J. Chem. Crystallogr. 46, 411–420.
K. Binnemans (2009). Lanthanide-based luminescent hybrid materials. Chem. Rev. 109, 4283–4374.
B. Biswas, P. Raghavaiah, N. Aliaga-Alcalde, et al. (2010). Syntheses, crystal structures and properties of a new family of isostructural and isomorphous compounds of type [M(L)(NCS)3] [M = La, Gd, Tb and Dy; L = a neutral hexadentate Schiff base]. Polyhedron. 29, 2716–2721.
D. Lionetti, V. W. Day, and J. D. Blakemore (2017). Noncovalent immobilization and surface characterization of lanthanide complexes on carbon electrodes. Dalton Trans. 46, 11779–11789.
Y. Zhang, M. Bhadbhade, L. Kong, et al. (2017). Syntheses and crystal structures of thorium(IV) and uranium (IV) tripodal metalloligands. Polyhedron. 138, 82–87.
H.-L. Wang, Z.-H. Zhu, X.-F. Ma, H.-H. Zou, and F.-P. Liang (2019). Metal-helix frameworks formed by μ3-NO3− with different orientations and connected to a heterometallic CuII10DyIII2 folded cluster. Chem. Eur. J. 25, 10813–10817.
H.-L. Wang, X.-F. Ma, Z.-H. Zhu, Y.-Q. Zhang, H.-H. Zou, and F.-P. Liang (2019). A series of dysprosium-based hydrogen-bonded organic frameworks (Dy-HOFs): thermally triggered off→on conversion of a single-ion magnet. Inorg. Chem. Front. 6, 2906–2913.
S. Alvarez, P. Alemany, D. Casanova, J. Cirera, M. Llunell, and D. Avnir (2005). Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 249, 1693–1708.
F.-S. Guo, A. K. Bar, and R. A. Layfield (2019). Main group chemistry at the interface with molecular magnetism. Chem. Rev. 119, 8479–8505.
N. Ishikawa, M. Sugita, T. Ishikawa, S. Koshihara, and Y. Kaizu (2003). Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J. Am. Chem. Soc. 125, 8694–8695.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22061005), Guangxi Natural Science Foundation (Grant Nos. 2020GXNSFAA159075, 2020AA23001AA), and Hebei Key Laboratory of Heterocyclic Compounds (Grant No. 19-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, TC., Bai, J., Sun, XH. et al. Lanthanide-Base Helical Chain Constructed by In Situ Schiff Base Reaction: Structures and Magnetic Properties. J Clust Sci 33, 2399–2406 (2022). https://doi.org/10.1007/s10876-021-02163-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-02163-9