Abstract
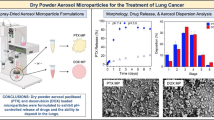
Lung cancer has become an immediate concern and reviewing the side effects associated with conventional chemotherapy, with the advent of nanobiomedicine, various nanoformulations are being researched. In the current study, our team has investigated aerosolized dry inhaler powder loaded with a combination of two potent anti-cancer drugs, cisplatin and paclitaxel for locoregional delivery of the same to the lung tissues. This nanoformulation has been investigated by morphological analysis and in-vitro studies to ensure proper therapeutics. Its efficacy has been determined by the anti-tumor studies. The size of the DPIs have been found to be in the range of 60–85 nm small enough to escape the clearance mechanisms in the pulmonary route. The nanoformulations with size < 200 nm easily move to the tumor vasculature which has been exploited completely here. The fine particle fraction (< 5 µm) deposited in the lungs was found to be 50%. They were also associated with reduced toxicity and they efficiently reduced the tumor volume by 75%. Therefore we may safely conclude that the current formulation overcomes the striking hindrances of nanocomposites namely increased toxicity, higher clearance from system and low efficacy due to inefficient targeting of drug.









Similar content being viewed by others
Data Availability
The data is provided with the manuscript.
References
T. Zhang, Y. Chen, Y. Ge, Y. Hu, M. Li, and Y. Jin (2018). Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm. Sin. B 8, 440–448.
J. Ahmad, S. Akhter, M. Rizwanullah, S. Amin, M. Rahman, M. Z. Ahmad, M. A. Rizvi, M. A. Kamal, and F. J. Ahmad (2015). Nanotechnology-based inhalation treatments for lung cancer: state of the art. Nanotechnol Sci. Appl. 8, 55–59.
R. Liu, S. Wei, J. Chen, and S. Xu (2014). Mesenchymal stem cells in lung cancer tumor microenvironment: their biological properties, influence on tumor growth and therapeutic implications. Cancer Lett. 353, 145–152.
Y. Chen, J. Li, S. Chen, Y. Zhang, Y. Hu, G. Zhang, X. Yan, and S. Jiao (2017). Nab-paclitaxel in combination with cisplatin versus docetaxel plus cisplatin as first-line therapy in non-small cell lung cancer. Sci. Rep. 7, 10760.
K. Wang, M. Chen, and W. Wu (2017). Analysis of microRNA (miRNA) expression profiles reveals 11 key biomarkers associated with non-small cell lung cancer. World J Surg Oncol. 15, 175.
S. V. Fulzele, A. Chatterjee, M. S. Shaik, T. Jackson, and M. Singh (2006). Inhalation delivery and anti-tumor activity of celecoxib in human orthotopic non-small cell lung cancer xenograft model. Pharm. Res. 23, 2094–2106.
W. H. Lee, C. Y. Loo, D. Traini, and P. M. Young (2015). Inhalation of nanoparticle-based drug for lung cancer treatment: advantages and challenges. Asian J. Pharm. Sci 10, 481–489.
A. B. Sandler, J. Nemunaitis, C. Denham, J. Von Pawel, Y. Cormier, U. Gatzemeier, K. Mattson, C. Manegold, M. Palmer, and A. Gregor (2000). Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non–small-cell lung cancer. J Clin Oncol. 18, 122–128.
J. H. Schiller, D. Harrington, C. P. Belani, C. Langer, A. Sandler, J. Krook, J. Zhu, and D. H. Johnson (2002). Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med. 346, 92–98.
A. J. Wozniak, J. J. Crowley, S. P. Balcerzak, G. R. Weiss, C. H. Spiridonidis, L. H. Baker, K. S. Albain, K. Kelly, S. A. Taylor, and D. R. Gandara (1998). Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: a Southwest Oncology Group study. J Clin Oncol. 16, 2459–2465.
S. Mangal, W. Gao, T. Li, and Q. T. Zhou (2017). Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: challenges and opportunities. Acta Pharm. Sin. 38, 782–797.
C. A. Ruge, J. Kirch, and C. M. Lehr (2013). Pulmonary drug delivery from generating aerosols to overcoming biological barriers—therapeutic possibilities and technological challenges. Lancet Respir Med. 1, 402–413.
E. Karathanasis, A. L. Ayyagari, R. Bhavane, R. V. Bellamkonda, and A. V. Annapragada (2005). Preparation of in vivo cleavable agglomerated liposomes suitable for modulated pulmonary drug delivery. J Control Release 103, 159–175.
J. C. Sung, B. L. Pulliam, and D. A. Edwards (2007). Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 25, 563–570.
A. Fels and Z. Cohn (1986). The alveolar macrophage. J. Appl. Physiol. 60, 353–369.
A. B. Dhanikula and R. Panchagnula (1999). Localized paclitaxel delivery. Int. J. Pharm. 183, 85–100.
M. B. Dolovich and R. Dhand (2011). Aerosol drug delivery: developments in device design and clinical use. The Lancet 377, 1032–1045.
R. Rosière, T. Berghmans, P. De Vuyst, K. Amighi, and N. Wauthoz (2019). The position of inhaled chemotherapy in the care of patients with lung tumors: Clinical feasibility and indications according to recent pharmaceutical progresses. Cancers 11, 329–335.
V. Levet, R. Rosière, R. Merlos, L. Fusaro, G. Berger, K. Amighi, and N. Wauthoz (2016). Development of controlled-release cisplatin dry powders for inhalation against lung cancers. Int. J. Pharm. 515, 209–220.
S. A. Meenach, K. W. Anderson, J. Z. Hilt, R. C. McGarry, and H. M. Mansour (2014). High-performing dry powder inhalers of paclitaxel DPPC/DPPG lung surfactant-mimic multifunctional particles in lung cancer: physicochemical characterization, in vitro aerosol dispersion, and cellular studies. AAPS PharmSciTech. 15, 1574–1587.
N. Wauthoz, P. Deleuze, A. Saumet, C. Duret, R. Kiss, and K. Amighi (2011). Temozolomide-based dry powder formulations for lung tumor-related inhalation treatment. Pharm. Res. 28, 762–775.
F. Gagnadoux, A. Pape, E. Lemarie, S. Lerondel, I. Valo, V. Leblond, J. Racineux, and T. Urban (2005). Aerosol delivery of chemotherapy in an orthotopic model of lung cancer. Eur Respir J. 26, 657–661.
Kondo K., Fujino H., Kimura J., Takizawa H., Sawada N., Miyoshi T., Sakiyama S., Monden Y. Aerosol therapy with liposome-encapsulated cisplatin for lung cancer in orthotopically implanted SCID mice. In.: AACR; 2004.
Y. Zou, H. Fu, S. Ghosh, D. Farquhar, and J. Klostergaard (2004). Antitumor activity of hydrophilic paclitaxel copolymer prodrug using locoregional delivery in human orthotopic non–small cell lung cancer xenograft models. Clin Cancer Res. 10, 7382–7391.
Y. Wei, J. Liang, X. Zheng, C. Pi, H. Liu, H. Yang, Y. Zou, Y. Ye, and L. Zhao (2017). Lung-targeting drug delivery system of baicalin-loaded nanoliposomes: development, biodistribution in rabbits, and pharmacodynamics in nude mice bearing orthotopic human lung cancer. Int J Nano. 12, 251.
M. Malamatari, S. Somavarapu, M. Bloxham, and G. Buckton (2015). Nanoparticle agglomerates of indomethacin: the role of poloxamers and matrix former on their dissolution and aerosolisation efficiency. Int. J. Pharm. 495, 516–526.
R. Pradhan, M. Mandal, A. Mitra, and S. Das (2014). Monitoring cellular activities of cancer cells using impedance sensing devices. Sens Actuators B: Chemical 193, 478–483.
Z. Zhang, H. Wang, Q. Ding, Y. Xing, Z. Xu, C. Lu, D. Luo, L. Xu, W. Xia, and C. Zhou (2018). Establishment of patient-derived tumor spheroids for non-small cell lung cancer. PloS one 13, e0194016.
C. P. Dora, S. K. Singh, S. Kumar, A. K. Datusalia, and A. Deep (2010). Development and characterization of nanoparticles of glibenclamide by solvent displacement method. Acta Pol Pharm. 67, 283–290.
Á. Farkas, F. Lizal, J. Jedelsky, J. Elcner, A. Horváth, and M. Jicha (2019). Simulation of airway deposition of an aerosol drug in COPD patients. Pharmaceutics 11, 153.
J. Treat, N. Damjanov, C. Huang, S. Zrada, and A. Rahman (2001). Liposomal-encapsulated chemotherapy: preliminary results of a phase I study of a novel liposomal paclitaxel. Oncology (Williston Park, NY) 15, 44–48.
A. Cabanes, K. E. Briggs, P. C. Gokhale, J. Treat, and A. Rahman (1998). Comparative in vivo studies with paclitaxel and liposome-encapsulated paclitaxel. Int. J. Oncol. 12, 1035–1075.
D. M. Nguyen, D. Lorang, G. A. Chen, J. H. Stewart IV., E. Tabibi, and D. S. Schrump (2001). Enhancement of paclitaxel-mediated cytotoxicity in lung cancer cells by 17-allylamino geldanamycin: in vitro and in vivo analysis. Ann Thorac Surg. 72, 371–379.
Author information
Authors and Affiliations
Contributions
Conceptualization: JZ; Methodology: JZ, LL, JY, ZC; Formal analysis and Investigation: LL, JY, ZC; Writing (Original draft preparation) LL; JZ Writing—review & editing: JZ. The manuscript has been read and approved by all the authors, that the requirements for authorship as stated earlier in this document have been met, and that each author believes that the manuscript represents honest work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests.
Ethical approval
The institutional small animal ethics committee had approved the study vide ethical approval nr. 56–2-10/2018. All animal studies have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent for publication
The authors give their consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, L., Yu, J., Chen, Z. et al. Improved Primary Lung Carcinoma Therapeutics Utilizing a Non-Invasive Approach of Combinatorial Drug Loaded Aerosolized Dry Inhaler Powder. J Clust Sci 33, 1781–1791 (2022). https://doi.org/10.1007/s10876-021-02103-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-02103-7