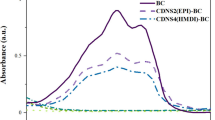
Abstract
Amygdalin is an herbal cyanoglycoside that has an anti-tumor effect. The utilization of this compound encounters various challenges results from releasing hydrogen cyanide. Apparently, the nano-formulation approach can increase its therapeutic effects. The β-cyclodextrin (β-CD) as a cyclic oligosaccharide is widely used in drug delivery. In this study, we aimed to nano-formulate the amygdalin by β-cyclodextrin in order to increase its effect on MCF-7 cell line. The synthesized β-CD-Amygdalin nanoparticle size, surface morphology, and chemical structure were determined by dynamic light scattering (DLS), Scanning electron microscopy (SEM), and Fourier transform infrared spectroscopy (FT-IR), respectively. Also, we calculated the drug loading (DL), entrapment efficiency (EE), and drug releases. The MTT assay, wound-healing assay, real-time PCR, and flow cytometry assays were carried out to evaluate the β-CD-Amygdalin and amygdalin effects on cell viability, migration, expression of migration-related genes, and apoptosis, respectively. The results showed that the nanoparticle synthesized in the mean diameter of 54.94 nm with − 27.9 mV zeta potential, uniformity of shape, and expected structure. The DL and EE values were calculated in 17.5% and 90%. A slow curve of the amygdalin release profile with two burst release times in 6 h and 48 h was observed. The cellular and molecular evaluation of β-CD-Amygdalin and amygdalin effects on MCF-7 cells revealed that the β-CD-Amygdalin had greater effects than amygdalin. In conclusion, these results suggest that the nanoformulation of amygdalin with β-CD may elevate amygdalin therapeutic efficacy.









Similar content being viewed by others
Data availability
Hereby we state that data sharing is not applicable in our submission.
References
P. Liczbinski and B. Bukowska (2018). Molecular mechanism of amygdalin action in vitro: review of the latest research. Immunopharmacol. Immunotoxicol. 40 (3), 212–218. https://doi.org/10.1080/08923973.2018.1441301.
Z. Zdrojewicz, A. Otlewska, P. Hackemer, and A. Otlewska (2015). Amygdalin—structure and clinical significance. Polski merkuriusz lekarski 38 (227), 300–303.
V. Jaswal, J. Palanivelu, and C. Ramalingam (2018). Effects of the Gut microbiota on Amygdalin and its use as an anti-cancer therapy: Substantial review on the key components involved in altering dose efficacy and toxicity. Biochem. Biophys. Rep. 14, 125–132. https://doi.org/10.1016/j.bbrep.2018.04.008.
S. Xu, X. Xu, S. Yuan, H. Liu, M. Liu, Y. Zhang, H. Zhang, Y. Gao, R. Lin, and X. Li (2017). Identification and analysis of amygdalin, neoamygdalin and amygdalin amide in different processed bitter almonds by HPLC-ESI-MS/MS and HPLC-DAD. Molecules 22 (9), 1425. https://doi.org/10.3390/molecules22091425.
M. F. Wahab, Z. S. Breitbach, D. W. Armstrong, R. Strattan, and A. Berthod (2015). Problems and pitfalls in the analysis of amygdalin and its epimer. J. Agric. Food Chem. 63 (40), 8966–8973. https://doi.org/10.1021/acs.jafc.5b03120.
B. Mosayyebi, M. Imani, L. Mohammadi, A. Akbarzadeh, N. Zarghami, M. Edalati, E. Alizadeh, and M. Rahmati (2020). An update on the toxicity of cyanogenic glycosides bioactive compounds: possible clinical application in targeted cancer therapy. Mater. Chem. Phys. 246, 122841. https://doi.org/10.1016/j.matchemphys.2020.122841.
H. Sauer, C. Wollny, I. Oster, E. Tutdibi, L. Gortner, S. Gottschling, and S. Meyer (2015). Severe cyanide poisoning from an alternative medicine treatment with amygdalin and apricot kernels in a 4-year-old child. Wiener medizinische Wochenschrift 165 (9–10), 185–188. https://doi.org/10.1007/s10354-014-0340-7.
T. Dang, C. Nguyen, and P. N. Tran (2017). Physician beware: severe cyanide toxicity from amygdalin tablets ingestion. Case Rep. Emerg. Med. 35, 4289527. https://doi.org/10.1155/2017/4289527.
C. G. Moertel, M. M. Ames, J. S. Kovach, T. P. Moyer, J. R. Rubin, and J. H. Tinker (1981). A pharmacologic and toxicological study of amygdalin. Jama 245 (6), 591–594.
X. Y. He, L. J. Wu, W. X. Wang, P. J. Xie, Y. H. Chen, and F. Wang (2020). Amygdalin—a pharmacological and toxicological review. J. Ethnopharmacol. 254, 112717. https://doi.org/10.1016/j.jep.2020.112717.
C. C. Bai, B. R. Tian, T. Zhao, Q. Huang, and Z. Z. Wang (2017). Cyclodextrin-catalyzed organic synthesis: reactions, mechanisms, and applications. Molecules 22 (9), 1475. https://doi.org/10.3390/molecules22091475.
H. Leemhuis, R. M. Kelly, and L. Dijkhuizen (2010). Engineering of cyclodextrin glucanotransferases and the impact for biotechnological applications. Appl. Microbiol. Biotechnol. 85 (4), 823–835. https://doi.org/10.1007/s00253-009-2221-3.
F. Topuz and T. Uyar (2018). Electrospinning of cyclodextrin functional nanofibers for drug delivery applications. Pharmaceutics 11 (1), 6. https://doi.org/10.3390/pharmaceutics11010006.
B. Gidwani and A. Vyas (2015). A Comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Biomed. Res. Int. 21, 198268–198268. https://doi.org/10.1155/2015/198268.
A. J. S. P. Rasheed (2008). Cyclodextrins as drug carrier molecule: a review. Sci. Pharm. 76 (4), 567–598.
E. Abbasi, A. Akbarzadeh, M. Kouhi, and M. Milani (2016). Graphene: synthesis, bio-applications, and properties. Artif. Cells Nanomed. Biotechnol. 44 (1), 150–156. https://doi.org/10.3109/21691401.2014.927880.
F. Ahmadi-Aghkand, S. Gholizadeh-Ghaleh Aziz, Y. Panahi, H. Daraee, F. Gorjikhah, S. Gholizadeh-Ghaleh Aziz, A. Hsanzadeh, and A. J. A. C. Akbarzadeh (2016). Biotechnology recent prospective of nanofiber scaffolds fabrication approaches for skin regeneration. Nanomedicine 44 (7), 1635–1641.
M. Saleem, J. Asif, M. Asif, and U. Saleem (2018). Amygdalin from apricot kernels induces apoptosis and causes cell cycle arrest in cancer cells: an updated review. Anti-Cancer Agents Med. Chem. 18 (12), 1650–1655. https://doi.org/10.2174/1871520618666180105161136.
J. Shi, Q. Chen, M. Xu, Q. Xia, T. Zheng, J. Teng, M. Li, and L. Fan (2019). Recent updates and future perspectives about amygdalin as a potential anticancer agent: a review. Cancer Med. 8 (6), 3004–3011. https://doi.org/10.1002/cam4.2197.
T. Dang, C. Nguyen, and P. N. Tran (2017). Physician beware: severe cyanide toxicity from amygdalin tablets ingestion. Case Rep. Emerg. Med. 2017, 4289527–4289527. https://doi.org/10.1155/2017/4289527.
J. Bromley, B. G. Hughes, D. C. Leong, and N. A. Buckley (2005). Life-threatening interaction between complementary medicines: cyanide toxicity following ingestion of amygdalin and vitamin C. Ann. Pharmacother. 39 (9), 1566–1569. https://doi.org/10.1345/aph.1E634.
A. Kumar and C. K. Dixit, 3—Methods for characterization of nanoparticles, in S. Nimesh, R. Chandra, and N. Gupta (eds.), Advances in nanomedicine for the delivery of therapeutic nucleic acids (Woodhead Publishing, Cambridge, 2017), pp. 43–58.
K. P. Sambasevam, S. Mohamad, N. M. Sarih, and N. A. Ismail (2013). Synthesis and characterization of the inclusion complex of β-cyclodextrin and azomethine. Int. J. Mol. Sci. 14 (2), 3671–3682. https://doi.org/10.3390/ijms14023671.
V. Webber, D. de Siqueira Ferreira, P. L. M. Barreto, V. Weiss-Angeli, and R. Vanderlinde (2018). Preparation and characterization of microparticles of β-cyclodextrin/glutathione and chitosan/glutathione obtained by spray-drying. Food Res. Int. (Ottawa, Ont) 105, 432–439. https://doi.org/10.1016/j.foodres.2017.11.035.
I. M. Savic, V. D. Nikolic, I. M. Savic-Gajic, L. B. Nikolic, S. R. Ibric, and D. G. Gajic (2015). Optimization of technological procedure for amygdalin isolation from plum seeds (Pruni domesticae semen). Front. Plant Sci. 6, 276. https://doi.org/10.3389/fpls.2015.00276.
A. Hedges, Chapter 22—cyclodextrins: properties and applications, in J. BeMiller and R. Whistler (eds.), Starch, 3rd ed. (Academic Press, San Diego, 2009), pp. 833–851.
M. J. Vazquez-Mellado, V. Monjaras-Embriz, and L. Rocha-Zavaleta, Chapter fourteen—erythropoietin, stem cell factor, and cancer cell migration, in G. Litwack (ed.), Vitamins and hormones, vol. 105 (Academic Press, New York, 2017), pp. 273–296.
S. Kurosaka and A. Kashina (2008). Cell biology of embryonic migration. Birth Defects Res C Embryo Today 84 (2), 102–122. https://doi.org/10.1002/bdrc.20125.
L. Li, Y. He, M. Zhao, and J. Jiang (2013). Collective cell migration: implications for wound healing and cancer invasion. Burns Trauma 1 (1), 21–26. https://doi.org/10.4103/2321-3868.113331.
J. Mani, J. Neuschäfer, C. Resch, J. Rutz, S. Maxeiner, F. Roos, F. K. Chun, E. Juengel, and R. A. Blaheta (2020). Amygdalin modulates prostate cancer cell adhesion and migration in vitro. Nutr. Cancer 72 (3), 528–537. https://doi.org/10.1080/01635581.2019.1637442.
J. Makarević, J. Rutz, E. Juengel, S. Kaulfuss, I. Tsaur, K. Nelson, J. Pfitzenmaier, A. Haferkamp, and R. A. Blaheta (2014). Amygdalin influences bladder cancer cell adhesion and invasion in vitro. PLoS ONE 9 (10), e110244–e110244. https://doi.org/10.1371/journal.pone.0110244.
J.-L. Maître and C.-P. Heisenberg (2013). Three functions of cadherins in cell adhesion. Curr. Biol. 23 (14), R626–R633. https://doi.org/10.1016/j.cub.2013.06.019.
A. Jeanes, C. J. Gottardi, and A. S. Yap (2008). Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene 27 (55), 6920–6929. https://doi.org/10.1038/onc.2008.343.
A. M. Mendonsa, T.-Y. Na, and B. M. Gumbiner (2018). E-cadherin in contact inhibition and cancer. Oncogene 37 (35), 4769–4780. https://doi.org/10.1038/s41388-018-0304-2.
K. M. Mrozik, O. W. Blaschuk, C. M. Cheong, A. C. W. Zannettino, and K. Vandyke (2018). N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 18 (1), 939–939. https://doi.org/10.1186/s12885-018-4845-0.
X. Liu, P. Su, S. Meng, M. Aschner, Y. Cao, W. Luo, G. Zheng, and M. Liu (2017). Role of matrix metalloproteinase-2/9 (MMP2/9) in lead-induced changes in an in vitro blood-brain barrier model. Int. J. Biol. Sci. 13 (11), 1351–1360. https://doi.org/10.7150/ijbs.20670.
L. Qian, B. Xie, Y. Wang, and J. Qian (2015). Amygdalin-mediated inhibition of non-small cell lung cancer cell invasion in vitro. Int. J. Clin. Exp. Pathol. 8 (5), 5363–5370.
Z. Wang, K. Fang, G. Wang, X. Guan, Z. Pang, Y. Guo, Y. Yuan, N. Ran, Y. Liu, and F. Wang (2019). Protective effect of amygdalin on epithelial-mesenchymal transformation in experimental chronic obstructive pulmonary disease mice. Phytother. Res. 33 (3), 808–817. https://doi.org/10.1002/ptr.6274.
Acknowledgement
This manuscript was supported by Tabriz University of Medical Sciences. Authors would like to thank Tabriz University of Medical Sciences for financial supporting of this project (Grant Number 61976).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mosayyebi, B., Imani, M., Mohammadi, L. et al. Comparison Between β-Cyclodextrin-Amygdalin Nanoparticle and Amygdalin Effects on Migration and Apoptosis of MCF-7 Breast Cancer Cell Line. J Clust Sci 33, 935–947 (2022). https://doi.org/10.1007/s10876-021-02019-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-02019-2