Abstract
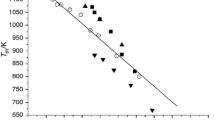
While phase diagrams for metal nanoalloys are important to know the conditions at which a nanoparticle keeps a particular shape and kind of surface, experiments to build phase diagrams at the nanoscale are difficult to implement; a posible alternative is the use of atomistic simulations. In this work, a set of molecular dynamics simulations is implemented to build the phase diagram of icosahedral, cuboctahedral, and decahedral AuCu nanoparticles of 2 to 4 nm in size, with several relative concentrations of the metals, using the quantum-corrected version of the Sutton and Chen interaction model. In the obtained phase diagrams, the congruent melting point shifts towards high concentrations of copper in accordance with previous theoretical results, and the local density mapping and bond-order analysis as function of temperature show that for the largest particles, partial premelting of the surface coexists with a pseudo-crystalline region that shrinks as the temperature is incremented, followed by the nucleation of a melted region in the inner volume of the particle that grows until the particle melts as a whole. After melting, it is found that gold has a tendency to migrate to the surface, but the alloy remains in the whole volume of the nanoparticle.






Similar content being viewed by others
References
Ackland, G.J., Jones, A.P.: Applications of local crystal structure measures in experiment and simulation. Physical Review B 73(5), 4655–7 (2006).
Aguado, A., Jarrold, M.F.: Melting and Freezing of Metal Clusters. Annual Review of Physical Chemistry 62(1), 151–172 (2011).
Alarifi, H.A., Atis, M., Ouml zdo gbreve an, C., Hu, A., Yavuz, M., Zhou, Y.: Molecular Dynamics Simulation of Sintering and Surface Premelting of Silver Nanoparticles. Materials Transactions 54(6), 884–889 (2013)
An, K., Somorjai, G.A.: Size and Shape Control of Metal Nanoparticles for Reaction Selectivity in Catalysis. ChemCatChem 4(10), 1512–1524 (2012).
Bajaj, S., Haverty, M.G., Arróyave, R., Goddard III FRSC, W.A., Shankar, S.: Phase stability in nanoscale material systems: extension from bulk phase diagrams. Nanoscale 7(21), 9868–9877 (2015)
Baker, H., others: ASM Handbook: Alloy Phase Diagrams, Vol. 3, vol. 3. ASM International, Metals Park, OH, USA (1992)
Çagin, T., Kimura, Y., Qi, Y., Li, H., Ikeda, H., Johnsonb, W.L., Goddard, W.A.: Calculation of Mechanical, Thermodynamic and Transport Properties of Metallic Glass Formers. MRS Proceedings 554, 43 (1998).
Chakravarty, C., Debenedetti, P.G., Stillinger, F.H.: Lindemann measures for the solid-liquid phase transition. The Journal of Chemical Physics 126(20), 204508–10 (2007).
Chernyshev, A.P.: Melting of surface layers of nanoparticles: Landau model. Materials Chemistry and Physics 112(1), 226–229 (2008).
Chushak, Y., Bartell, L.: Molecular dynamics simulations of the freezing of gold nanoparticles. European Physical Journal D 16, 43–46 (2001).
Cleveland, C., Luedtke, W., Landman, U.: Melting of gold clusters. Physical Review B 60(7), 5065–5077 (1999).
Cuenya, B.R.: Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 518(12), 3127–3150 (2010).
Cui, M., Lu, H., Jiang, H., Cao, Z., Meng, X.: Phase Diagram of Continuous Binary Nanoalloys: Size, Shape, and Segregation Effects. Nature Publishing Group pp. 1–10 (2017)
Dai, C., Saidi, P., Song, H., Yao, Z., Daymond, M.R., Hoyt, J.J.: A test of a phenomenological model of size dependent melting in Au nanoparticles. Acta Materialia 136, 11–20 (2017).
Durán-Álvarez, J.C., Avella, E., Ramírez-Zamora, R.M., Zanella, R.: Photocatalytic degradation of ciprofloxacin using mono- (Au, Ag and Cu) and bi- (Au–Ag and Au–Cu) metallic nanoparticles supported on TiO2 under UV-C and simulated sunlight. Catalysis Today 266, 175–187 (2016).
Gong, J.: Structure and Surface Chemistry of Gold-Based Model Catalysts. Chemical Reviews 112(5), 2987–3054 (2012).
Guisbiers, G., Mejía-Rosales, S., Khanal, S., Ruiz-Zepeda, F., Whetten, R.L., José-Yacamán, M.: Gold-Copper Nano-Alloy, “Tumbaga”, in the Era of Nano: Phase Diagram and Segregation. Nano Letters 14(11), 6718–6726 (2014).
Hill, T.L.: Perspective: Nanothermodynamics. Nano Letters 1(3), 111–112 (2001).
Honeycutt, J.D., Andersen, H.C.: Molecular dynamics study of melting and freezing of small Lennard-Jones clusters. The Journal of Physical Chemistry 91(19), 4950–4963 (1987).
Joshi, A.M., Tucker, M.H., Delgass, W.N., Thomson, K.T.: CO adsorption on pure and binary-alloy gold clusters: A quantum chemical study. The Journal of Chemical Physics 125(19), 194707–12 (2006).
Lindemann, F.A.: Uber die berechnung molekularer Eigenfrequenzen. Physikalische Zeitschrift 11, 609–612 (1910).
Luo, S.N., Ahrens, T.J., Çağın, T., Strachan, A., Goddard, W.A., Swift, D.C.: Maximum superheating and undercooling: Systematics, molecular dynamics simulations, and dynamic experiments. Physical Review B 68(13), 75–11 (2003).
Martin, T.P., Naher, U., Schaber, H., Zimmermann, U.: Evidence for a size-dependent melting of sodium clusters. The Journal of Chemical Physics 100(3), 2322–2324 (1994).
Mejía-Rosales, S.J., Fernández-Navarro, C., Pérez-Tijerina, E., Montejano-Carrizales, J.M., José-Yacamán, M.: Two-Stage Melting of Au-Pd Nanoparticles. The Journal of Physical Chemistry B 110(26), 12884–12889 (2006).
Montejano-Carrizales, J.M., Iñiguez, M.P., Alonso, J.A., López, M.J.: Theoretical study of icosahedral Ni clusters within the embedded-atom method. Physical Review B 54(8), 5961–5969 (1996).
Monzó, J., Malewski, Y., Kortlever, R., Vidal-Iglesias, F.J., Solla-Gullón, J., Koper, M.T.M., Rodriguez, P.: Enhanced electrocatalytic activity of Au@Cu core@shell nanoparticles towards CO2 reduction. Journal of Materials Chemistry A: Materials for energy and sustainability 3(47), 23690–23698 (2015).
Rafii-Tabar, H., Sutton, A.P.: Long-range Finnis-Sinclair potentials for f.c.c. metallic alloys. Philosophical Magazine Letters 63(4), 217–224 (1991)
Rodríguez-Proenza, C., Palomares-Báez, J., Chávez-Rojo, M., García-Ruiz, A., Azanza-Ricardo, C., Santoveña-Uribe, A., Luna-Bárcenas, G., Rodríguez-López, J., Esparza, R.: Atomic Surface Segregation and Structural Characterization of PdPt Bimetallic Nanoparticles. Materials 11(10), 1882–15 (2018).
Shibuta, Y., Suzuki, T.: Melting and solidification point of fcc-metal nanoparticles with respect to particle size: A molecular dynamics study. Chemical Physics Letters 498(4–6), 323–327 (2010).
Skriver, H.L., Rosengaard, N.M.: Surface energy and work function of elemental metals. Physical Review B 46(11), 7157–7168 (1992).
Sutton, A.P., Chen, J.: Long-range finnis-sinclair potentials. Philosophical Magazine Letters 61(3), 139–146 (1990).
Taherkhani, F., Akbarzadeh, H., Feyzi, M., Rafiee, H.R.: Disorder effect on heat capacity, self-diffusion coefficient, and choosing best potential model for melting temperature, in gold–copper bimetallic nanocluster with 55 atoms. Journal of Nanoparticle Research 17(1), 16–10 (2015).
Todorov, I.T., Smith, W., Trachenko, K., Dove, M.T.: DL\_POLY\_3: new dimensions in molecular dynamics simulations via massive parallelism. Journal of Materials Chemistry 16(20), 1911–8 (2006).
Tran, R., Xu, Z., Radhakrishnan, B., Winston, D., data, W.S.S., 2016: Surface energies of elemental crystals. nature.com/scientificdata 3(1), 238 (2016)
Vitos, L., Ruban, A.V., Skriver, H.L., Kollar, J.: The surface energy of metals. Surface Science 411(1–2), 186–202 (1998).
Wen, Y.H., Zhang, Y., Zheng, J.C., Zhu, Z.Z., Sun, S.G.: Orientation-Dependent Structural Transition and Melting of Au Nanowires. The Journal of Physical Chemistry C 113(48), 20611–20617 (2009).
Yang, C.C., Mai, Y.W.: Thermodynamics at the nanoscale: A new approach to the investigation of unique physicochemical properties of nanomaterials. Materials Science and Engineering: R: Reports 79, 1–40 (2014).
Yin, J., Shan, S., Yang, L., Mott, D., Malis, O., Petkov, V., Cai, F., Shan Ng, M., Luo, J., Chen, B.H., Engelhard, M., Zhong, C.J.: Gold-Copper Nanoparticles: Nanostructural Evolution and Bifunctional Catalytic Sites. Chemistry of Materials 24(24), 4662–4674 (2012).
Zhang, L., Kim, H.Y., Henkelman, G.: CO Oxidation at the Au–Cu Interface of Bimetallic Nanoclusters Supported on CeO 2(111). The Journal of Physical Chemistry Letters 4(17), 2943–2947 (2013).
Acknowledgements
The authors thank the support from UANL through the PAICYT grants CE335-15 and CE878-19, and from the Laboratorio Nacional de Supercómputo del Sureste de México for computing time.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martínez-Muñoz, H.R., Mejía-Rosales, S. The AuCu Phase Diagram at the Nano Scale: A Molecular Dynamics Approach. J Clust Sci 33, 785–793 (2022). https://doi.org/10.1007/s10876-021-02015-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-02015-6