Abstract
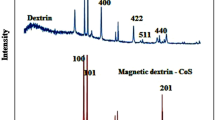
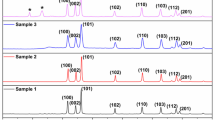
In this study, a recyclable antimicrobial agent against infectious pathogens in contaminated water was proposed. Iron oxide (IO) loaded poly (methyl methacrylate) (PMMA) core/polyethyleneimine (PEI) shell nanoparticles (PMMA/PEI-IO) was fabricated. The PMMA/PEI-IO showed a cationic surface charge property which is reflected by the protonation behavior of PEI. IO content in PMMA/PEI was evaluated by TGA and indicated that the residue incorporation of IO was 50% by dried weight. The existing of IO in PMMA/PEI was confirmed by the vibrating-sample magnetometer (VSM) showing a superparamagnetic property. The antibacterial ability was confirmed in waterborne bacteria, Escherichia coli and Staphylococcus aureus. The disturbance of the cell membrane integrity occurred after 20 min post-incubation of PMMA/PEI-IO with bacteria. Antimicrobial activity and recycling ability was further confirmed in an infectious, zoonotic and waterborne pathogen, Streptococcus agalactiae. Antiviral activity of PMMA/PEI-IO was observed and proved in an avian flu virus H3N2, a virus that has the potential to contaminate water reservoirs. The result showed that PMMA/PEI-IO nanoparticles were effective in killing H3N2. This study proposes a novel nanoparticle-based antimicrobial agents with recycle ability. Moreover, PMMA/PEI-IO nanoparticle is a potent bactericidal and virucidal agents when tested with waterborn zoonotic pathogens.




Similar content being viewed by others
References
A. Fenwick (2006). Waterborne infectious diseases–could they be consigned to history? Science 313 (5790), 1077–1081. https://doi.org/10.1126/science.1127184.
“WHO | Guidelines for drinking-water quality, 3rd edition: Volume 1 - Recommendations,” WHO. http://www.who.int/water_sanitation_health/publications/gdwq3rev/en/ (accessed Mar. 09, 2020).
A. E. Dalziel, S. Delean, S. Heinrich, and P. Cassey (2016). Persistence of low pathogenic influenza A virus in water: a systematic review and quantitative meta-analysis. PLoS ONE 11 (10), e0161929. https://doi.org/10.1371/journal.pone.0161929.
J. K. Dunnick and R. L. Melnick (1993). Assessment of the carcinogenic potential of chlorinated water: experimental studies of chlorine, chloramine, and trihalomethanes. J. Natl. Cancer Inst. 85 (10), 817–822. https://doi.org/10.1093/jnci/85.10.817.
H. Komulainen (2004). Experimental cancer studies of chlorinated by-products. Toxicology 198 (1–3), 239–248. https://doi.org/10.1016/j.tox.2004.01.031.
Y. Cao, M. Naseri, Y. He, C. Xu, L. J. Walsh, and Z. M. Ziora (2019). Non-antibiotic antimicrobial agents to combat biofilm-forming bacteria. J Glob Antimicrob Res. https://doi.org/10.1016/j.jgar.2019.11.012.
S. D. Lakshmi, P. K. Avti, and G. Hegde (2018). Activated carbon nanoparticles from biowaste as new generation antimicrobial agents: a review. Nano-Struct. Nano-Objects 16, 306–321. https://doi.org/10.1016/j.nanoso.2018.08.001.
R. León, et al. (2020). Exploring small cationic peptides of different origin as potential antimicrobial agents in aquaculture. Fish Shellfish Immunol. 98, 720–727. https://doi.org/10.1016/j.fsi.2019.11.019.
M. R. P. K. Muraleedaran and V. M. A. Mujeeb (2015). Applications of chitosan powder with in situ synthesized nano ZnO particles as an antimicrobial agent. Int. J. Biol. Macromol. 77, 266–272. https://doi.org/10.1016/j.ijbiomac.2015.03.058.
M. Hasanin, A. El-Henawy, W. H. Eisa, H. El-Saied, and M. Sameeh (2019). Nano-amino acid cellulose derivatives: eco-synthesis, characterization, and antimicrobial properties. Int. J. Biol. Macromol. 132, 963–969. https://doi.org/10.1016/j.ijbiomac.2019.04.024.
X. Qu, P. J. J. Alvarez, and Q. Li (2013). Applications of nanotechnology in water and wastewater treatment. Water Res. 47 (12), 3931–3946. https://doi.org/10.1016/j.watres.2012.09.058.
T. Matsunaga, R. Tomoda, T. Nakajima, N. Nakamura, and T. Komine (1988). Continuous-sterilization system that uses photosemiconductor powders. Appl. Environ. Microbiol. 54 (6), 1330–1333.
Q. L. Feng, J. Wu, G. Q. Chen, F. Z. Cui, T. N. Kim, and J. O. Kim (2000). A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 52 (4), 662–668. https://doi.org/10.1002/1097-4636(20001215)52:4%3c662::aid-jbm10%3e3.0.co;2-3.
S. Kang, M. Pinault, L. D. Pfefferle, and M. Elimelech (2007). Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 23 (17), 8670–8673. https://doi.org/10.1021/la701067r.
M. J. Gallagher, H. Huang, K. J. Schwab, D. H. Fairbrother, and B. Teychene (2013). Generating backwashable carbon nanotube mats on the inner surface of polymeric hollow fiber membranes. J. Membr. Sci. 446, 59–67. https://doi.org/10.1016/j.memsci.2013.06.015.
H. K. No, N. Young Park, S. Ho Lee, and S. P. Meyers (2002). Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 74 (1), 65–72. https://doi.org/10.1016/S0168-1605(01)00717-6.
H. Kusic, D. Leszczynska, N. Koprivanac, and I. Peternel (2011). Role of quantum dots nanoparticles in the chemical treatment of colored wastewater: catalysts or additional pollutants. J. Environ. Sci. 23 (9), 1479–1485. https://doi.org/10.1016/S1001-0742(10)60609-2.
S. Galdiero, A. Falanga, M. Vitiello, M. Cantisani, V. Marra, and M. Galdiero (2011). Silver nanoparticles as potential antiviral agents. Molecules 16 (10), 8894–8918. https://doi.org/10.3390/molecules16108894.
M. Rai, S. D. Deshmukh, A. P. Ingle, I. R. Gupta, M. Galdiero, and S. Galdiero (2016). Metal nanoparticles: the protective nanoshield against virus infection. Crit. Rev. Microbiol. 42 (1), 46–56. https://doi.org/10.3109/1040841X.2013.879849.
R. Surudžić, et al. (2016). Physico–chemical and mechanical properties and antibacterial activity of silver/poly(vinyl alcohol)/graphene nanocomposites obtained by electrochemical method. Compos. Part B 85, 102–112. https://doi.org/10.1016/j.compositesb.2015.09.029.
I.-G. Athanasoulia, M. Mikropoulou, S. Karapati, P. Tarantili, and C. Trapalis (2018). Study of thermomechanical and antibacterial properties of TiO2/Poly(lactic acid) nanocomposites. Mater. Today 5 (14), 27553–27562. https://doi.org/10.1016/j.matpr.2018.09.075.
Y.-X. Hou, H. Abdullah, D.-H. Kuo, S.-J. Leu, N. S. Gultom, and C.-H. Su (2018). A comparison study of SiO2/nano metal oxide composite sphere for antibacterial application. Compos B 133, 166–176. https://doi.org/10.1016/j.compositesb.2017.09.021.
K.-S. Huang, C.-H. Yang, S.-L. Huang, C.-Y. Chen, Y.-Y. Lu, and Y.-S. Lin (2016). Recent advances in antimicrobial polymers: a mini-review. Int J Mol Sci. https://doi.org/10.3390/ijms17091578.
W. A. Rutala and D. J. Weber (2013). Disinfection and sterilization: an overview. Am. J. Infect. Control 41 (5 Suppl), S2-5. https://doi.org/10.1016/j.ajic.2012.11.005.
F. Hossain, J. Perales-Perez Oscar, S. Hwang, and F. Román (2014). Antimicrobial nanomaterials as water disinfectant: applications, limitations and future perspectives. Sci. Total Environ. 466–467, 1047–1059. https://doi.org/10.1016/j.scitotenv.2013.08.009.
A. J. Huh and Y. J. Kwon (2011). ‘Nanoantibiotics’: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control Release 156 (2), 128–145. https://doi.org/10.1016/j.jconrel.2011.07.002.
M. Neu, D. Fischer, and T. Kissel (2005). Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J. Gene Med. 7 (8), 992–1009. https://doi.org/10.1002/jgm.773.
U. Lungwitz, M. Breunig, T. Blunk, and A. Göpferich (2005). Polyethylenimine-based non-viral gene delivery systems. Eur. J. Pharm. Biopharm. 60 (2), 247–266. https://doi.org/10.1016/j.ejpb.2004.11.011.
N. Saengkrit, et al. (2012). The PEI-introduced CS shell/PMMA core nanoparticle for silencing the expression of E6/E7 oncogenes in human cervical cells. Carbohydr. Polym. 90 (3), 1323–1329. https://doi.org/10.1016/j.carbpol.2012.06.079.
S. A. Koplin, S. Lin, and T. Domanski (2008). Evaluation of the antimicrobial activity of cationic polyethylenimines on dry surfaces. Biotechnol. Prog. 24 (5), 1160–1165. https://doi.org/10.1002/btpr.32.
N. Hasan, et al. (2019). PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater. Sci. Eng. C 103, 109741. https://doi.org/10.1016/j.msec.2019.109741.
J. Haldar, D. An, L. Álvarez de Cienfuegos, J. Chen, and A. M. Klibanov (2006). Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proc. Natl. Acad. Sci. USA 103 (47), 17667–17671. https://doi.org/10.1073/pnas.0608803103.
N. Kawabata and M. Nishiguchi (1988). Antibacterial activity of soluble pyridinium-type polymers. Appl. Environ. Microbiol. 54 (10), 2532–2535.
H. Khalil, T. Chen, R. Riffon, R. Wang, and Z. Wang (2008). Synergy between polyethylenimine and different families of antibiotics against a resistant clinical isolate of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52 (5), 1635–1641. https://doi.org/10.1128/AAC.01071-07.
M. Ratanajanchai, S. Soodvilai, N. Pimpha, and P. Sunintaboon (2014). Polyethylenimine-immobilized core–shell nanoparticles: synthesis, characterization, and biocompatibility test. Mater. Sci. Eng. C 34, 377–383. https://doi.org/10.1016/j.msec.2013.09.037.
S. Mornet, S. Vasseur, F. Grasset, and E. Duguet (2004). Magnetic nanoparticle design for medical diagnosis and therapy. J. Mater. Chem. 14 (14), 2161–2175. https://doi.org/10.1039/B402025A.
O. Veiseh, J. W. Gunn, and M. Zhang (2010). Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 62 (3), 284–304. https://doi.org/10.1016/j.addr.2009.11.002.
J. Yang, J. Gunn, S. R. Dave, M. Zhang, Y. A. Wang, and X. Gao (2008). Ultrasensitive detection and molecular imaging with magnetic nanoparticles. Analyst 133 (2), 154–160. https://doi.org/10.1039/b700091j.
L. J. Reed, H. Muench, H. A. Muench, L. I. Reed, L. I. Reed, and L. J. Reed, A simple method of estimating fifty percent endpoints. 1938, Accessed March 09, 2020. https://www.scienceopen.com/document?vid=21401620-94ad-4100-a3e1-56741c541ab6.
T. Xia, et al. (2009). Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano 3 (10), 3273–3286. https://doi.org/10.1021/nn900918w.
J. D. Ziebarth and Y. Wang (2010). Understanding the protonation behavior of linear polyethylenimine in solutions through Monte Carlo simulations. Biomacromolecules 11 (1), 29–38. https://doi.org/10.1021/bm900842d.
K. Gibney, et al. (2012). Poly(ethylene imine)s as antimicrobial agents with selective activity. Macromol. Biosci. 12 (9), 1279–1289. https://doi.org/10.1002/mabi.201200052.
L. Zhou, B. He, and F. Zhang (2012). Facile one-pot synthesis of iron oxide nanoparticles cross-linked magnetic poly(vinyl alcohol) gel beads for drug delivery. ACS Appl. Mater. Interfaces 4 (1), 192–199. https://doi.org/10.1021/am201649b.
S. Saesoo, et al. (2018). Characterization of liposome-containing SPIONs conjugated with anti-CD20 developed as a novel theranostic agent for central nervous system lymphoma. Colloids Surf. B Biointerfaces 161, 497–507. https://doi.org/10.1016/j.colsurfb.2017.11.003.
C. Wiegand, M. Bauer, U.-C. Hipler, and D. Fischer (2013). Poly(ethyleneimines) in dermal applications: biocompatibility and antimicrobial effects. Int. J. Pharm. 456 (1), 165–174. https://doi.org/10.1016/j.ijpharm.2013.08.001.
Y.-M. Chen, Y.-C. Chung, L. W. Wang, K.-T. Chen, and S.-Y. Li (2002). Antibacterial properties of chitosan in waterborne pathogen. J. Environ. Sci. Health Part A 37 (7), 1379–1390. https://doi.org/10.1081/ESE-120005993.
R. M. Epand and R. F. Epand (2009). Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochimica et Biophysica Acta (BBA) - Biomembranes 1788 (1), 289–294. https://doi.org/10.1016/j.bbamem.2008.08.023.
R. M. Epand, C. Walker, R. F. Epand, and N. A. Magarvey (2016). Molecular mechanisms of membrane targeting antibiotics. Biochimica et Biophysica Acta (BBA) - Biomembranes 1858 (5), 980–987. https://doi.org/10.1016/j.bbamem.2015.10.018.
I. Stock and B. Wiedemann (1999). Natural antibiotic susceptibility of Escherichia coli, Shigella, E. vulneris, and E. hermannii strains. Diagn. Microbiol. Infect. Dis. 33 (3), 187–199. https://doi.org/10.1016/S0732-8893(98)00146-1.
H. Nogueira, S. Toma, A. Silveira Jr., and K. Araki (2020). Zeolite-SPION nanocomposite for ammonium and heavy metals removal from wastewater. J Braz Chem Soc. https://doi.org/10.21577/0103-5053.20200097.
A. Kapri, M. G. H. Zaidi, A. Satlewal, and R. Goel (2010). SPION-accelerated biodegradation of low-density polyethylene by indigenous microbial consortium. Int. Biodeterior Biodegrad 64, 238–244. https://doi.org/10.1016/j.ibiod.2010.02.002.
N. Mon-On, W. Surachetpong, S. Mongkolsuk, and K. Sirikanchana (2018). Roles of water quality and disinfectant application on inactivation of fish pathogenic Streptococcus agalactiae with povidone iodine, quaternary ammonium compounds and glutaraldehyde. J. Fish Dis. 41 (5), 783–789. https://doi.org/10.1111/jfd.12776.
S. Shigematsu, et al. (2014). Influenza A virus survival in water is influenced by the origin species of the host cell. Influenza Other Respir. Viruses 8 (1), 123–130. https://doi.org/10.1111/irv.12179.
G. A. Spoden, et al. (2012). Polyethylenimine is a strong inhibitor of human papillomavirus and cytomegalovirus infection. Antimicrob. Agents Chemother. 56 (1), 75–82. https://doi.org/10.1128/AAC.05147-11.
M. Rossier, A. Schaetz, E. Athanassiou, R. Grass, and W. Stark (2011). Reversible As(V) adsorption on magnetic nanoparticles and pH dependent desorption concentrates dilute solutions and realizes true moving bed reactor systems. Chem. Eng. J. 175, 244–250. https://doi.org/10.1016/j.cej.2011.09.101.
C. Lu, H. Chiu, and H. Bai (2007). Comparisons of adsorbent cost for the removal of zinc(II) from aqueous solution by carbon nanotubes and activated carbon. J. Nanosci. Nanotechnol. 7, 1647–1652. https://doi.org/10.1166/jnn.2007.349.
Acknowledgements
This work has been supported by NANOTEC, NSTDA, Ministry of Higher Education, Science, Research and Innovation (MHESI), Thailand.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pimpha, N., Woramongkolchai, N., Sunintaboon, P. et al. Recyclable Iron Oxide Loaded Poly (Methyl Methacrylate) Core/Polyethyleneimine Shell Nanoparticle as Antimicrobial Nanomaterial for Zoonotic Pathogen Controls. J Clust Sci 33, 567–577 (2022). https://doi.org/10.1007/s10876-021-01990-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-01990-0