Abstract
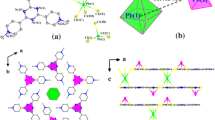
Interaction of [Pb(SiPr)2]n with 1.5 equiv. of AgNO3 in DMF under aerobic conditions at room temperature gave a cationic lead-centered thiolate cluster [Pb13(SiPr)6O8](NO3)4 (1). While treatment of [Pb(SiPr)2]n in refluxing DMF in air afforded a two-dimensional layered structure of [Pb14(SiPr)16O6]n (2), comprising of [Pb14(SiPr)18O6]2− units bridged the iPrS− moieties. Molecular structures of the two oxidation products of μ4-oxygen-bridged high-nuclearity lead(II) clusters have been determined by single-crystal X-ray analysis. The UV/Vis properties of the two clusters in both solid state and solution state were also investigated in this paper.
Graphic Abstract





Similar content being viewed by others
References
1. D. Fenske, C. Persau, S. Dehnen, and C. E. Anson (2004). Angew. Chem. Int. Ed. 43, 305–309.
2. Z. J. Wang, H. D. Ye, Y. G. Li, Y. Z. Li, and H. Yan (2013). J. Am. Chem. Soc. 135, 11289–11298.
3. M. S. Queen, B. D. Towey, K. A. Murray, B. S. Veldkamp, H. J. Byker, and R. K. Szilagyi (2013). Coord. Chem. Rev. 257, 564–578.
4. S.-J. Bao, C.-Y. Liu, M. Zhang, X.-R. Chen, H. Yu, H.-X. Li, P. Braunstein, and J.-P. Lang (2019). Coord. Chem. Rev. 397, 28–53.
5. F. Wang, F.-L. Li, M.-M. Xu, H. Yu, J.-G. Zhang, H.-T. Xia, and J.-P. Lang (2015). J. Mater. Chem. A 3, 5908–5916.
6. F. Wang, C.-Y. Ni, Q. Liu, F.-L. Li, J. Shi, H.-X. Li, and J.-P. Lang (2013). Chem. Commun. 49, 9248–9250.
7. A. L. P. Cornacchio, and N. D. Jones (2006). J. Mater. Chem. 16, 1171–1177.
8. G. G. Briand, A. D. Smith, G. Schatte, A. J. Rossini, and R. W. Schurko (2007). Inorg. Chem. 46, 8625–8637.
9. P. B. Hitchcock, M. F. Lappert, B. J. Samways, and E. L. Weinberg (1983). J. Chem. Soc., Chem. Comm. 1492–1494
10. P. B. Hitchcock, H. A. Jasim, R. E. Kelly, and M. F. Lappert (1985). J. Chem. Soc., Chem. Comm. 1778–1780
11. F. T. Edelmann, J.-K. F. Buijink, S. A. Brooker, R. Herbst-Irmer, U. Kilimann, and F. M. Bohnen (2000). Inorg. Chem. 39, 6134–6135.
12. A. Eichhöfer (2005). Eur. J. Inorg. Chem. 9, 1683–1688.
13. R. A. Shaw, M. Woods (1971). J. Chem. Soc. A 1569–1571.
SMART and SAINT+ for Windows NT Version 6.02a, Bruker Analytical X-ray Instruments Inc., Madison, Wisconsin, USA (1998).
15. G. M. Sheldrick, SADABS, University of Göttingen, Germany (1996).
G. M. Sheldrick, SHELXTL Software Reference Manual. Version 5.1, (Bruker AXS Inc, Madison, Wisconsin, USA, 1997).
17. G. M. Sheldrick (2008), Acta Crystallogr. A 64 112–122.
18. J. L. Liu, F. Hu, M. M. Sheng, A.-Q. Jia, and Q.-F. Zhang (2020), J. Cluster Sci. DOI: 10.1007/s10876-020-01872-x.
19. J. S. Magyar, T. C. Weng, C. M. Stern, D. F. Dye, and H. A. Godwin (2005). J. Am. Chem. Soc. 127, 9495–9505.
20. P. A. W. Dean, J. J. Vittal, and N. C. Payne (1984). Inorg. Chem. 23, 4232–4236.
21. P. Klüfers and J. Schuhmacher (1994). Angew. Chem. Int. ed. Engl. 33, 1863–1865.
22. P. Klüfers and J. Schuhmacher (1995). Z. Anorg. Allg. Chem. 621, 19–22.
23. M. Katz, H. Kaluarachchi, R. Batchelor, A. Bokov, Z. G. and Ye, D. Leznoff (2007). Angew. Chem. 46, 8720–8720.
24. A. Eichhöfer, O. Hampe, and M. Blom (2003). Eur. J. Inorg. Chem. 1307–1314
25. S. E. Appleton, G. G.Briand, A. Decken, and A. S. Smith (2004). Dalton Trans. 21, 3515–3520.
Acknowledgements
This project was supported by Natural Science Foundation of Anhui Province (2008085MB58) and Young Wanjiang Scholar program of Anhui Province.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qin, SS., Liu, JL., Hu, F. et al. Formation and Structures of the μ4-Oxygen-Bridged High-Nuclearity Lead(II) Clusters from a Lead Propane-2-thiolate Complex. J Clust Sci 32, 1593–1599 (2021). https://doi.org/10.1007/s10876-020-01917-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01917-1