Abstract
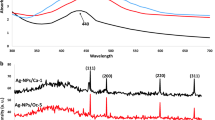
The present study reports the synthesis of silver nanoparticles (AgNPs) using haloalkaliphilic Streptomyces spp. characterization, and antifungal activity thereof. The UV visible spectra of synthesized AgNPs showed a characteristic absorption peak at 430 nm, due to the excitation of Surface Plasmon Resonance. Scanning electron microscopy and transmission electron microscopy images showed spherical shape NPs with an average particle size of 16.4 ± 2.2 nm. The crystalline structure of the AgNPs was confirmed by X-ray diffraction (XRD). Zeta potential analyses revealed that NPs were negatively charged (− 8.12 ± 3.87 mV). The synthesized AgNPs are significantly active against phytopathogenic fungi, Fusarium verticillioides and Ustilago maydis. Microscopic, histo- and bio-chemical investigation of AgNPs against F. verticillioides revealed that AgNPs at 100 μg concentration inhibits the hyphal growth and conidia germination, and ~ 42.85% reduction of ergosterol biosynthesis. The results of propidium iodide staining and high relative cell membrane conductivity confirmed AgNPs mediated damage to the membrane. Moreover, the AgNPs synthesized by Streptomyces spp. inhibit the growth of F. verticillioides could be due to the inhibition of ergosterol biosynthesis and membrane damage. In our knowledge, this is the first report demonstrating the anti F. verticillioides activity of AgNPs synthesized by Streptomyces spp.







Similar content being viewed by others
References
U. Basavaraj, N. Praveenkumar, S. Sabiha, S. Rupali, and B. Samprita (2012). Int. J. Pharm. Bio Sci. 2, 10–14.
J. Ahire and L. Dicks (2016). Curr. Microbiol. 73, 236–241.
F. Chen, M. Ding, X. Wang, and L. Shao (2004). Biomaterials 25, 723–727.
S. Aminabad, M. Farshbaf, and A. Akbarzadeh (2019). Cell Biochem. Biophys. 77, 123–137.
T. Wang, L. Yang, B. Zhang, and J. Liu (2010). Colloid Surf. B 80, 94–102.
D. Muhammad and R. Rida (2017). Anal. Lett. 50, 50–62.
F. Gholami, H. Mosmeri, M. Shavandi, M. Dastgheib, and A. Amoozegar (2019). Sci. Total Environ. 655, 633–640.
R. Rao, U. Kulkarni, J. Thomas, and P. Edwards (2000). Chem. Soc. Rev. 29, 27–35.
M. Rai, A. Yadav, and A. Gade (2009). Biotechnol. Adv. 27, 76–83.
S. Zhao, M. Du, and Y. Tian (2012). World J. Microbiol. Biotechnol. 28, 2919–2927.
A. Ahmad, P. Mukherjee, S. Senapati, D. Mandal, I. Khan, R. Kumar, and M. Sastry (2003). Colloids Surf. B 28, 313–318.
N. Kharat and D. Mendhulkar (2016). Mat. Sci. Eng. C 62, 719–724.
P. Manivasagan, V. Jayachandran, S. Kalimuthu, S. Kannan, and K. Se-Kwon (2013). Biomed. Res. Int. 1–9.
S. Kim, K. Houng, L. Hyun, and J. Kye (1991). J. Microbiol. Biotechnol. 1, 288–292.
Y. Tsibakhashvili, I. Kirkesali, T. Pataraya, A. Gurielidze, L. Kalabegishvili, N. Gvarjaladze, et al. (2011). Adv. Sci. Lett. 4, 3408–3417.
S. Sadhasivam, P. Shanmugam, and K. Yun (2010). Colloids Surf. B 81, 358–362.
L. Karthik, G. Kumar, V. Kirthi, A. Rahuman, and B. Rao (2014). Bioproc. Biosyst. Eng. 37, 261–267.
M. Wypij, J. Czarnecka, M. Świecimska, H. Dahm, M. Rai, and P. Golinska (2018). World J. Microbiol. Biotechnol. 34, 23 (1–13).
G. Agrios Plant Pathology, 5th ed (Elsevier Academic Press, San Diego, 2005).
A. Dhekney, T. Li, M. Van Aman, M. Dutt, J. Tattersall, T. Kelley, and J. Gray (2005). Fruit Crops Tropical Species 738, 743–748.
N. Krishnan, B. Velramar, and K. Velu (2019). Pest Biochem. Physiol. 155, 101–107.
W. Bacon, E. Yates, M. Hinton, and F. Meredith (2001). Environ. Health Perspect 109, 325–332.
J. Ahire, D. Neveling, and L. Dicks (2015). Curr. Microbiol. 71, 24–30.
A. Sidhu, S. Ghatelwal, K. Gumber, and A. Bala (2017). Appl. Nanosci. 7, 617–623.
A. Petica, S. Gavriliu, M. Lungu, N. Buruntea, and C. Panzaru (2008). Mater Sci Eng B 152, 22–27.
W. Kim, S. Kim, K. Lamsal, J. Kim, B. Kim, M. Jung, and S. Lee (2009). J. Microbiol. Biotechnol. 19, 760–764.
K. Marathe, S. Kasar, A. Chaudhari, and V. Maheshwari (2016). Proc. Biochem. 51, 1650–1663.
K. Marathe, A. Chaudhari, K. Kamalaja, and V. Maheshwari (2015). Biocatal. Agric. Biotechnol. 5, 58–68.
R. Sharma, D. Acharya, S. Moghe, B. Shrivastava, M. Gangrade, T. Shripathi, and V. Ganesan (2014). Mat. Sci. Semicon. Proc. 23, 42–49.
M. Elamawi, E. Al-Harbi, and A. Hendi (2018). Egypt J. Biol. Pest Co. 28, 28.
H. Hassouni, I. Alaoui, K. Lamrani, G. Perraud, C. Augur, and S. Roussos (2007). Micol. Aplicada Int. 19, 7–14.
T. Gao, H. Zhou, W. Zhou, L. Hu, J. Chen, and Z. Shi (2016). Molecules 21, 770.
Y. Duan, C. Ge, S. Liu, C. Chen, and M. Zhou (2013). Pest Biochem. Physiol. 106, 61–67.
H. Firstencel, M. Butt, and I. Carruther (1990). J Invertebr Pathol 55, 258–264.
A. Arthington-Skaggs, H. Jradi, T. Desai, and J. Morrison (1999). J. Clin. Microbiol. 37, 3332–3337.
A. Bose, H. Keharia, and P. Deshpande (2013). Chin. Phys. Lett. 30, 128103.
X. Wei, M. Luo, W. Li, L. Yang, X. Liang, et al. (2012). Bioresour Technol 103, 273–278.
S. Pirtarighat, M. Ghannadnia, and S. Baghshahi (2019). J. Nanostruct. Chem. 9, 1–9.
X. Zhao, L. Yan, X. Xu, H. Zhao, Y. Lu, Y. Wang, et al. (2019). Appl. Microbiol. Biotechnol. 103, 1–4.
S. Eppler, G. Rupprechter, A. Anderson, and A. Somorjai (2000). J Phys Chem B 104, 7286–7292.
A. Omran, N. Nassar, A. Younis, A. Fatthallah, A. Hamdy, H. Shatoury, and N. El- Gendy (2018). J. Appl. Microbiol. 126, 138-154.
S. Prakasham, K. Buddana, K. Yannam, and S. Guntuku (2012). J. Micobiol. Biotechnol. 22, 614–621.
P. Balashanmugam, D. Balakumaran, R. Murugan, K. Dhanapal, and T. Kalaichelvan (2016). Microbiol. Res. 192, 52–64.
A. Richards, V. Veses, and A. Gow (2010). Fung Biol. Rev. 24, 93–105.
A. Amro, P. Kotra, K. Wadu-Mesthrige, A. Bulychev, S. Mobashery, and Y. Liu (2000). Langmuir 16, 2789–2796.
L. Elechiguerra, L. Burt, R. Morones, A. Camacho-Bragado, X. Gao, H. Lara, and J. Yacaman (2005). J. Nanobiotechnol. 3–6.
K. Lamsal, W. Kim, H. Jung, S. Kim, S. Kim, and S. Lee (2011). Mycobiol 39, 194–199.
A. Aguilar-Méndez, E. San Martín-Martínez, L. Ortega-Arroyo, G. Cobián-Portillo, and E. Sánchez-Espíndola (2011). J. Nanopart. Res. 13, 2525–2532.
V. Mahdizadeh, N. Safaie, and F. Khelghatibana (2015). J. Crop. Prot. 4, 291–300.
M. Khatami, N. Zafarnia, H. Bami, I. Sharifi, and H. Singh (2018). J. Mycol. Med. 28, 37–644.
A. Roy, O. Bulut, K. Mandal, and D. Yilmaz (2019). RSC Adv. 9, 2673–2702.
J. Du, Z. Hu, Z. Yu, H. Li, J. Pan, D. Zhao, and Y. Bai (2019). Mat. Sci. Eng. C 102, 247–253.
Z. Xu General Plant Pathology (Higher Education Press, Beijing, 2009). (in Chinese).
Acknowledgements
KRM acknowledges SERB (Science and Engineering Research Board, Govt. of India) for providing N-PDF (File No. N-PDF/2017/0000115 dated 6/10/2017). Authors are thankful to Dr. Bhardwaj, Jain Irrigation System Ltd., Jalgaon, India for kind help with fluorescent microscopy. Authors are also grateful to University Grants Commission, New Delhi and Department of Science and Technology, Govt. of India for making the research facilities available under the UGC-SAP and DST-FIST programs sanctioned at both places, the School of Life Sciences and the University Institute of Chemical Technology, KBCNMU, Jalgaon.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marathe, K., Naik, J. & Maheshwari, V. Biogenic Synthesis of Silver Nanoparticles Using Streptomyces spp. and their Antifungal Activity Against Fusarium verticillioides. J Clust Sci 32, 1299–1309 (2021). https://doi.org/10.1007/s10876-020-01894-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01894-5