Abstract
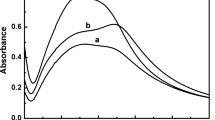
An environmentally benign solvothermal process was introduced to synthesize monodisperse silver nanoparticles (Ag NPs). Silver nitrate (AgNO3) was reduced by ethanol which was also used for recyclable solvent. The as-synthesized Ag NPs were stabilized by oleate. Effects of reaction temperature, reaction time, AgNO3 concentration as well as molar ratio of sodium oleate and AgNO3 on the formation and surface plasmon resonance (SPR) were determined by means of UV–Vis spectrophotometer. TEM and XRD were used to characterize the size, morphology and crystalline structure of Ag NPs. The excessive AgNO3 over sodium oleate and high concentration of AgNO3 are favorable for the synthesis of monodisperse Ag NPs in large scale. The characteristic SPR absorption of Ag NPs centers at ca. 430 nm despite of reaction temperature, reaction time and even AgNO3 concentration. The nucleation and growth of Ag NPs completed in a very short period of time, showing the high efficiency of our synthetic route for the synthesis of uniform Ag NPs.








Similar content being viewed by others
References
J. Natsuki and T. Abe (2011). J. Colloid Interf. Sci. 359, 19.
Y. Xia, X. Xia, Y. Wang, and S. Xie (2013). Mrs Bull. 38, 335.
C. C. M. Neumann, E. Laborda, K. Tschulik, K. R. Ward, and R. G. Compton (2013). Nano Res. 6, 511.
R. Sahraei, J. Cheraghi, R. Hushmandfar, S. Abbasi, S. S. Mortazavi, H. Noorizadeh, and A. Farmany (2012). J. Chin. Chem. Soc. 60, 195.
E. A. Terenteva, V. V. Apyari, E. V. Kochuk, S. G. Dmitrienko, and Y. A. Zolotov (2017). J. Anal. Chem. 72, 1138.
B. Ruttkay-Nedecky, S. Skalickova, M. Kepinska, K. Cihalova, M. Docekalova, M. Stankova, D. Uhlirova, C. Fernandez, J. Sochor, H. Milnerowicz, M. Beklova, and R. Kizek (2019). J. Nanosci. Nanotechnol. 19, 2762.
C. Marambio-Jones and E. M. V. Hoek (2010). J. Nanopart. Res. 12, 1531.
Y. Yang, S. Matsubara, L. Xiong, T. Hayakawa, and M. Nogami (2007). J. Phys. Chem. C 111, 9095.
B. W. Yang, Z. Y. Guo, Z. M. Liu, M. M. Wan, and X. C. Qin (2013). Spectrosc. Spect. Anal. 33, 1816.
D. S. Ahlawat, R. Kumari, and I. Yadav (2014). Int. J. Nanosci. 13, 1450004.
J. P. Ge, W. Chen, L. P. Liu, and Y. D. Li (2006). Chem-eur J. 12, 6552.
X. Wang, J. Zhuang, Q. Peng, and Y. D. Li (2005). Nature 437, 121.
H. Xu and K. S. Suslick (2010). ACS Nano 4, 3209.
M. Ider, K. Abderrafi, A. Eddahbi, S. Ouaskit, and A. Kassiba (2017). J. Clust. Sci. 28, 1051.
A. Pal, S. Shah, and S. Devi (2009). Mater. Chem. Phys. 114, 530.
O. A. Boryak, M. V. Kosevich, V. V. Chagovets, and V. S. Shelkovsky (2017). J. Anal. Chem. 72, 1289.
J. Helmlinger, M. Heise, M. Heggen, M. Ruck, and M. Epple (2015). RSC Adv. 5, 92144.
M. N. Nadagouda, T. F. Speth, and R. S. Varma (2011). Acc. Chem. Res. 44, 469.
E. P. Marta, B. M. Klaus, G. Marina, H. Zsuzsanna, K. Janina, and K. Katrin (2016). Beilstein J. Nanotechnol. 7, 834.
K. Gudikandula and S. C. Maringanti (2016). J. Exp. Nanosci. 11, 714.
S. Perni, V. Hakala, and P. Prokopovich (2014). Colloid Surface A 460, 219.
S. Iravani (2011). Green Chem. 13, 2638.
K. N. Thakkar, S. S. Mhatre, and R. Y. Parikh (2010). Nanomed. Nanotechnol. 6, 257.
S. Chen, H. B. Lee, and R. L. Penn (2019). J. Phys. Chem. C 123, 12444.
Z. Leng, D. Wu, Q. Yang, S. Zeng, and W. Xia (2018). Optik 154, 33.
A. I. Titkov, E. Y. Gerasimov, M. V. Shashkov, O. A. Logutenko, N. V. Bulina, Y. M. Yukhin, and N. Z. Lyakhov (2016). Colloid J. 78, 515.
X. Gao, D. Li, Z. Chen, X. Mei, and Y. Wang (2016). New J. Chem. 40, 7265.
Y. Zhang, R. Shi, and P. Yang (2014). J. Nanosci. Nanotechnol. 14, 3011.
G. Prozorova, A. Pozdnyakov, N. Kuznetsova, S. Korzhova, A. Emelyanov, T. Ermakova, T. Y. Fadeeva, and L. Sosedova (2014). Int. J. Nanomed. 9, 1883.
Z. Huang, H. Jiang, P. Liu, J. Sun, D. Guo, J. Shan, and N. Gu (2015). J. Mater. Chem. A 3, 1925.
T. K. Sau and A. L. Rogach (2010). Adv. Mater. 22, 1781.
I. A. Wani, S. Khatoon, A. Ganguly, J. Ahmed, A. K. Ganguli, and T. Ahmad (2010). Mater. Res. Bull. 45, 1033.
G. Vanitha, K. Rajavel, G. Boopathy, V. Veeravazhuthi, and P. Neelamegam (2016). Synth. React. Inorg. M. 47, 761.
D. Wei, W. Sun, W. Qian, Y. Ye, and X. Ma (2009). Carbohyd. Res. 344, 2375.
C. Tian, B. Mao, E. Wang, Z. Kang, Y. Song, C. Wang, S. Li, and L. Xu (2007). Nanotechnology 18, 285607.
Z. Xu and G. Hu (2012). RSC Adv. 2, 11404.
K. M. Koczkur, S. Mourdikoudis, L. Polavarapu, and S. E. Skrabalak (2015). Dalton T. 44, 17883.
D. Wang and Y. Li (2011). Inorg. Chem. 50, 5196.
H. H. Lin and K. M. Chi (2011). J. Nanosci. Nanotechnol. 11, 1193.
A. Sarkar, S. Kapoor, and T. Mukherjee (2010). Res. Chem. Intermediat. 36, 411.
Y. M. Chen and H. W. Jia (2014). Mater. Lett. 132, 389.
Acknowledgement
This work was supported by the Natural Science Foundation of Hunan Province (2017JJ3264).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, J., Chen, Y. & Nie, X. Environmentally Benign and Large-Scale Synthesis of Monodisperse Oleate-Protected Silver Nanoparticles in Ethanol. J Clust Sci 32, 899–905 (2021). https://doi.org/10.1007/s10876-020-01852-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01852-1