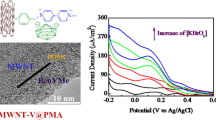
Abstract
Single wall carbon nanotube substituted ionic liquids were synthesized successfully by esterification reaction of the carboxylated single wall carbon nanotubes (SWNTs) with hydroxyl ammonium ionic liquids (ILs) of the formula CH3N(CH2CH2OH)2(CnH2n+1)Br (n = 4, 8, 12), which subsequently reacted with molybdophosphoric acid (PMo12) to form three new ternary composite materials (SWNTs-ILCn-PMo12) (n = 4, 8, 12). The composites modified glass carbon electrodes were used to study electrochemical properties systematically through cyclic voltammetry method. The results showed that electronic conductivity of SWNTs moiety and ionic conductivity of ILs moiety in the composite materials played important roles in the electrochemistry and electrocatalysis. The length of alkyl carbon chain of the ionic liquids in the composite materials was also found to influence the electrochemical properties. The experimental results also showed that all the composites modified electrode can catalytically reduce and detect IO3−, BrO3− and NO2− with very high efficiency (low detection limit, short response time and wide linear range).









Similar content being viewed by others
References
M. Galiński, A. Lewandowski, and I. Stępniak (2006). Electrochim. Acta 51, 5567.
R. D. Rogers and K. R. Seddon (2003). Science 302, 792.
A. P. Abbott, G. Capper, D. L. Davies, H. L. Munro, R. K. Rasheed, and V. Tambyrajah (2001). Chem. Commun. 19, 2010.
P. Wasserscheid and W. Keim (2000). Angew. Chem. Int. Ed. 39, 3772.
S. V. Dzyuba and R. A. Bartsch (2001). Chem. Commun. 16, 1466.
T. Hirashige, R. Hagiwara, and Y. Ito (2000). J. Fluorine Chem. 106, 205.
J. C. Xiao and J. N. M. Shreeve (2005). J. Org. Chem. 70, 3072.
X. H. Li, D. B. Zhao, Z. F. Fei, and L. F. Wang (2006). Sci. China Ser. B Chem. 36, 181.
A. Dolbecq, E. Dumas, C. R. Mayer, and P. Mialane (2010). Chem. Rev. 110, 6009.
Y. H. Sun, L. I. Xiao-Ping, Z. M. Mei, Y. Zhu, and L. Niu (2011). Chem. Res. Chin. Univ. 27, 6.
Y. W. Li, Y. G. Li, Y. H. Wang, X. J. Feng, Y. Lu, and E. B. Wang (2009). Inorg. Chem. 48, 6452.
G. Hou, L. Bi, B. Li, and L. Wu (2010). Inorg. Chem. 49, 6474.
R. Thangamuthu, Y. C. Pan, and S. M. Chen (2010). Electroanalysis. 22, 1812.
J. D. Kim, S. Hayashi, T. Mori, and I. Honma (2007). Electrochim. Acta 53, 963.
X. Wu, W. Wu, Q. Wu, and W. Yan (2017). Langmuir. 33, 4242.
L. Hu, D. S. Hecht, and G. Grüner (2010). Chem. Rev. 110, 5790.
A. Sun, J. Zheng, and Q. Sheng (2012). Electrochim. Acta 65, 64.
Y. Li, X. Liu, X. Liu, N. Mai, Y. Li, W. Wei, and Q. Cai (2011). B Biointerfaces 88, 402.
Y. Zhang, Y. Shen, J. Yuan, D. Han, Z. Wang, Q. Zhang, and L. Niu (2006). Angew. Chem. Int. Ed. 45, 5867.
B. Haghighi, H. Hamidi, and L. Gorton (2010). Electrochim. Acta 55, 4750.
B. K. Mishra, P. Mukherjee, S. Dash, S. Patel, and H. N. Pati (2009). Inorg. Chem. 39, 2529.
S. Roy, A. Dasgupta, and P. K. Das (2006). Langmuir 22, 4567.
L. Liu, Y. Qin, Z. X. Guo, and D. Zhu (2003). Carbon 41, 331.
E. Rogelhernández, G. Alonsonuñez, J. P. Camarena, H. Espinozagómez, G. Aguirre, F. Paraguaydelgado, and R. Somanathan (2011). J. Mex. Chem. Soc. 55, 7.
R. Claude, F. Michel, F. Raymonde, and T. Rene (1983). Inorg. Chem. 22, 207.
W. Guo, L. Xu, F. Li, B. Xu, Y. Yang, S. Liu, and Z. Sun (2010). Electrochim. Acta 55, 1523.
S. D. L. Cruz, M. D. Green, Y. Ye, Y. A. Elabd, T. E. Long, and K. I. Winey (2012). J. Polym. Sci. Part A: Polym. Chem. 50, 338.
Z. Xu, N. Gao, and S. Dong (2006). Talanta 68, 753.
J. Cheng, G. Sàghiszabó, J. A. T. And, and C. J. Miller (1996). J. Am. Chem. Soc. 118, 680.
H. O. Finklea and D. D. Hanshew (1992). J. Am. Chem. Soc. 114, 3173.
P. Wang, X. Wang, and G. Zhu (2000). Electroanalysis 12, 1493.
Z. Li, J. Chen, D. Pan, W. Tao, L. Nie, and S. Yao (2006). Electrochim. Acta 51, 4255.
T. Peter Laboratory Techniques in Electroanalytical Chemistry (Dekker, New York, 1984).
L. Guadagnini and D. Tonelli (2013). Sens. Actuators B Chem. 188, 806.
A. Salimi, A. Noorbakhsh, and M. Ghadermarzi (2006). Sens. Actuators B Chemical 1, 530.
A. Salimi, V. Alizadeh, and H. Hadadzadeh (2010). Electroanalysis 16, 1984.
W. Song, X. Chen, Y. Jiang, Y. Liu, C. Sun, and X. Wang (1999). Anal. Chim. Acta 394, 73.
L. Li, W. Li, C. Sun, and L. Li (2002). Electroanalysis 14, 368.
L. D. Li, W. J. Li, and C. Q. Li (2000). Chem. J. Chin. Univ. 21, 865.
T. Dong, F. Chen, J. Du, and C. Hu (2010). J. Cluster Sci. 21, 779.
L. Liu, S. Y. Song, and P. Y. Zhang (2012). Acta Phys. Chim. Sin. 28, 427.
Acknowledgements
The financial support of the Natural Science Foundation of China is greatly acknowledged. Prof. Xue Duan, Beijing University of Chemical Technology, is greatly acknowledged for his kind support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, T., Zhou, Q., Ren, X. et al. Incorporation of Keggin-Type Phosphomolybdic Acid, Ionic Liquid and Carbon Nanotube Leading to Formation of Multifunctional Ternary Composite Materials: Fabrication, Characterization and Electrochemical Reduction/Detection of Iodate, Borate and Nitrite. J Clust Sci 30, 973–984 (2019). https://doi.org/10.1007/s10876-019-01557-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-019-01557-0