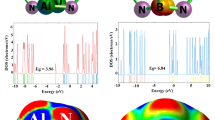
Abstract
Using density functional theory calculations, the adsorption behavior and electronic sensitivity of an Al12N12 nanocluster, and an AlN nanocone were investigated toward purinethol (PE) drug. The drug tends to adsorbs on the AlN nanocluster and nanocone via its N atom with adsorption energies about − 38.2 and − 23.4 kcal/mol, respectively. The AlN nanocluster suffers from a long recovery time of about 3.1 × 1011 s at 298 K, and cannot be used as a sensor for PE drug. But the electrical conductivity of the AlN nanocone is largely increased by the PE drug adsorption which makes it sensitive to the drug. Also, AlN nanocone benefits from a short recovery time of about 20.8 s at room temperature. Thus, it was concluded that the AlN nanocone may be a promising candidate for detection of PE drug. We also show that by increasing the percentage of Hartree–Fock exchange in the functional, the adsorption energy is increased and the sensitivity is decreased.






Similar content being viewed by others
References
S. Sahasranaman, D. Howard, and S. Roy (2008). Clinical pharmacology and pharmacogenetics of thiopurines. Eur. J. Clin. Pharmacol. 64, 753–767.
A. A. Ensafi and H. Karimi-Maleh (2012). Determination of 6-mercaptopurine in the presence of uric acid using modified multiwall carbon nanotubes-TiO2 as a voltammetric sensor. Drug Test. Anal. 4, 970–977.
Z. Chen, G. Zhang, X. Chen, J. Chen, J. Liu, and H. Yuan (2013). A fluorescence switch sensor for 6-mercaptopurine detection based on gold nanoparticles stabilized by biomacromolecule. Biosens. Bioelectron. 41, 844–847.
B.-Y. Lu, H. Li, H. Deng, Z. Xu, W.-S. Li, and H.-Y. Chen (2008). Voltammetric determination of 6-mercaptopurine using [Co (phen) 3] 3 +/MWNT modified graphite electrode. J. Electroanal. Chem. 621, 97–102.
L. Wang and Z. Zhang (2008). The study of oxidization fluorescence sensor with molecular imprinting polymer and its application for 6-mercaptopurine (6-MP) determination. Talanta 76, 768–771.
R. Boulieu and T. Dervieux (1999). High-performance liquid chromotographic determination of methyl 6-mercaptopurine nucleotides (Me6-MPN) in red blood cells: Analysis of Me6-MPN per se or Me6-MPN derivative? J. Chromatogr. B Biomed. Sci. Appl. 730, 273–274.
S. Bashiri, E. Vessally, A. Bekhradnia, A. Hosseinian, and L. Edjlali (2017). Utility of extrinsic [60] fullerenes as work function type sensors for amphetamine drug detection: DFT studies. Vacuum 136, 156–162.
H. Salimi, A. A. Peyghan, and M. Noei (2015). Adsorption of formic acid and formate anion on ZnO nanocage: A DFT study. J. Cluster Sci. 26, 609–621.
F. Behmagham, E. Vessally, B. Massoumi, A. Hosseinian, and L. Edjlali (2016). A computational study on the SO2 adsorption by the pristine, Al, and Si doped BN nanosheets. Superlattices Microstruct. 100, 350–357.
A. A. Peyghan, M. B. Tabar, and S. Yourdkhani (2013). A theoretical study of OH and OCH3 free radical adsorption on a nanosized tube of BC2N. J. Cluster Sci. 24, 1–10.
J. Beheshtian, M. T. Baei, Z. Bagheri, and A. A. Peyghan (2013). Carbon nitride nanotube as a sensor for alkali and alkaline earth cations. Appl. Surf. Sci. 264, 699–706.
A. Hosseinian, A. Bekhradnia, E. Vessally, L. Edjlali, and M. D. Esrafili (2017). A DFT study on the central-ring doped HBC nanographenes. J. Mol. Graph. Model. 73, 101–107.
A. A. Peyghan, M. T. Baei, S. Hashemian, and P. Torabi (2013) First principles calculations of electric field effect on the (6, 0) zigzag single-walled silicon carbide nanotube for use in nano-electronic circuits. J. Cluster Sci. 24, 591–604.
M. T. Baei, A. A. Peyghan, and Z. Bagheri (2013) Electronic, energetic, and geometric properties of methylene-functionalized C60. J. Cluster Sci. 24, 669–678.
A. A. Peyghan, M. T. Baei, and S. Hashemian (2013). ZnO nanocluster as a potential catalyst for dissociation of H2S molecule. J. Cluster Sci. 24, 341–347.
E. Vessally, S. A. Siadati, A. Hosseinian, and L. Edjlali (2017). Selective sensing of ozone and the chemically active gaseous species of the troposphere by using the C20 fullerene and graphene segment. Talanta 162, 505–510.
K. Nejati, E. Vessally, P. D. K. Nezhad, H. Mofid, and A. Bekhradnia (2017). The electronic response of pristine, Al and Si doped BC2N nanotubes to a cathinone molecule: Computational study. J. Phys. Chem. Solids 111, 238–244.
J. Beheshtian, A. A. Peyghan, and Z. Bagheri (2012). Nitrate adsorption by carbon nanotubes in the vacuum and aqueous phase. Monatshefte für Chemie/Chem. Mon. 143, 1623–1626.
M. T. Baei, A. A. Peyghan, and Z. Bagheri (2013). Selective adsorption behavior of BC2N nanotubes toward fluoride and chloride. Solid State Commun. 159, 8–12.
N. L. Hadipour, A. Ahmadi Peyghan, and H. Soleymanabadi (2015). Theoretical study on the Al-doped ZnO nanoclusters for CO chemical sensors. J. Phys. Chem. C 119, 6398–6404.
K. Nejati, A. Hosseinian, L. Edjlali, and E. Vessally (2017). The effect of structural curvature on the cell voltage of BN nanotube based Na-ion batteries. J. Mol. Liq. 229, 167–171.
L. Safari, E. Vessally, A. Bekhradnia, A. Hosseinian, and L. Edjlali (2017). A Density functional theory study of the sensitivity of two-dimensional BN nanosheet to nerve agents cyclosarin and tabun. Thin Solid Films 623, 157–163.
A. A. Peyghan and H. Soleymanabadi (2015). Computational study on ammonia adsorption on the X12Y12 nanoclusters (X = B, Al and Y = N, P). Curr. Sci. 108, 00113891.
J. Beheshtian, A. A. Peyghan, and Z. Bagheri (2013). Functionalization of BN nanosheet with N2H4 may be feasible in the presence of Stone–Wales defect. Struct. Chem. 24, 1565–1570.
M. Samadizadeh, S. F. Rastegar, and A. A. Peyghan (2015). F−, Cl−, Li+ and Na+ adsorption on AlN nanotube surface: A DFT study. Physica E 69, 75–80.
Z. Rostami and H. Soleymanabadi (2017). Investigation of phosgene adsorption behavior on aluminum nitride nanocones: Density functional study. J. Mol. Liq. 248, 473–478.
J. Beheshtian, A. A. Peyghan, Z. Bagheri, and M. Kamfiroozi (2012). Interaction of small molecules (NO, H2, N2, and CH4) with BN nanocluster surface. Struct. Chem. 23, 1567–1572.
Z. Bagheri and A. A. Peyghan (1008). DFT study of NO2 adsorption on the AlN nanocones. Comput. Theor. Chem. 2013, 20–26.
A. V. Moradi, A. A. Peyghan, S. Hashemian, and M. T. Baei (2012). Theoretical study of thiazole adsorption on the (6, 0) zigzag single-walled boron nitride nanotube. Bull. Korean Chem. Soc. 33, 3285–3292.
J. Beheshtian, H. Soleymanabadi, A. A. Peyghan, and Z. Bagheri (2012). A DFT study on the functionalization of a BN nanosheet with PC-X, (PC = phenyl carbamate, X = OCH3, CH3, NH2, NO2 and CN). Appl. Surf. Sci. 268, 436–441.
M. T. Baei, M. R. Taghartapeh, E. T. Lemeski, and A. Soltani (2014). A computational study of adenine, uracil, and cytosine adsorption upon AlN and BN nano-cages. Phys. B 444, 6–13.
J. Beheshtian, A. A. Peyghan, M. B. Tabar, and Z. Bagheri (2013). DFT study on the functionalization of a BN nanotube with sulfamide. Appl. Surf. Sci. 266, 182–187.
E. Vessally, F. Behmagham, B. Massoumi, A. Hosseinian, and L. Edjlali (2016). Carbon nanocone as an electronic sensor for HCl gas: Quantum chemical analysis. Vacuum 134, 40–47.
G. Wang, H. Yuan, A. Kuang, W. Hu, G. Zhang, and H. Chen (2014). High-capacity hydrogen storage in Li-decorated (AlN)n (n = 12, 24, 36) nanocages. Int. J. Hydrog. Energy 39, 3780–3789.
J. Beheshtian, A. A. Peyghan, and Z. Bagheri (2013). Sensing behavior of Al-rich AlN nanotube toward hydrogen cyanide. J. Mol. Model. 19, 2197–2203.
A. S. Rad and K. Ayub (2016). Detailed surface study of adsorbed nickel on Al12N12 nano-cage. Thin Solid Films 612, 179–185.
M. T. Baei, E. Tazikeh Lemeski, and A. Soltani (2017). DFT study of the adsorption of H2O2 inside and outside Al12N12 nano-cage. Russ. J. Phys. Chem. A 91, 1527–1534.
J. Beheshtian, A. Ahmadi Peyghan, and Z. Bagheri (2012). A first-principles study of H2S adsorption and dissociation on the AlN nanotube. Physica E 44, 1963–1968.
A. Soltani, A. Ahmadi Peyghan, and Z. Bagheri (2013). H2O2 adsorption on the BN and SiC nanotubes: A DFT study. Physica E 48, 176–180.
M. T. Baei, A. Soltani, and S. Hashemian (2016). Adsorption properties of hydrazine on pristine and Si-doped Al12N12 nano-cage. Phosphorus Sulfur Silicon Relat. Elem. 191, 702–708.
D. L. Strout (2000). Structure and stability of boron nitrides: Isomers of B12N12. J. Phys. Chem. A 104, 3364–3366.
R. Wang, D. Zhang, and C. Liu (2005). Theoretical prediction of a novel inorganic fullerene-like family of silicon–carbon materials. Chem. Phys. Lett. 411, 333–338.
H.-S. Wu, F.-Q. Zhang, X.-H. Xu, C.-J. Zhang, and H. Jiao (2003). Geometric and energetic aspects of aluminum nitride cages. J. Phys. Chem. A 107, 204–209.
Q. Wang, Q. Sun, P. Jena, and Y. Kawazoe (2009). Potential of AlN nanostructures as hydrogen storage materials. ACS Nano 3, 621–626.
J. Beheshtian, A. A. Peyghan, and Z. Bagheri (2012). Selective function of Al12N12 nano-cage towards NO and CO molecules. Comput. Mater. Sci. 62, 71–74.
C. Liu, Z. Hu, Q. Wu, X. Wang, Y. Chen, H. Sang, J. Zhu, S. Deng, and N. Xu (2005). Vaporsolid growth and characterization of aluminum nitride nanocones. J. Am. Chem. Soc. 127, 1318–1322.
Q. Wu, N. Liu, Y. Zhang, W. Qian, X. Wang, and Z. Hu (2015). Tuning the field emission properties of AlN nanocones by doping. J. Mater. Chem. C 3, 1113–1117.
M. Mirzaei, M. Yousefi, and M. Meskinfam (2012). Chemical shielding properties for BN, BP, AlN, and AlP nanocones: DFT studies. Superlattices Microstruct. 51, 809–813.
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T. L. Windus, M. Dupuis, and J. A. Montgomery (1993). J. Comp. Chem. 14, 1347–1363.
J. Beheshtian, A. A. Peyghan, and Z. Bagheri (2012). Detection of phosgene by Sc-doped BN nanotubes: A DFT study. Sens. Actuators B Chem. 171, 846–852.
M. A. Abdulsattar (2011). SiGe superlattice nanocrystal pure and doped with substitutional phosphorus single atom: Density functional theory study. Superlattices Microstruct. 50, 377–385.
A. Soltani, M. T. Baei, E. Tazikeh Lemeski, S. Kaveh, and H. Balakheyli (2015). A DFT study of 5-fluorouracil adsorption on the pure and doped BN nanotubes. J. Phys. Chem. Solids 86, 57–64.
S. Tomic, B. Montanari, and N. M. Harrison (2008). The group III–V’s semiconductor energy gaps predicted using the B3LYP hybrid functional. Physica E 40, 2125–2127.
N. O’Boyle, A. Tenderholt, and K. Langner (2008). Cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 29, 839–845.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, Al M. A. Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople (2008) Gaussian 03, revision C, 02.
R. Dennington, T. Keith, and J. Millam (2009) GaussView, version 5. Semichem Inc., Shawnee Mission.
A. Ahmadi Peyghan, N. Hadipour, and Z. Bagheri (2013). Effects of Al-doping and double-antisite defect on the adsorption of HCN on a BC2N nanotube: DFT studies. J. Phys. Chem. C 117, 2427–2432.
M. Eslami, V. Vahabi, and A. A. Peyghan (2016). Sensing properties of BN nanotube toward carcinogenic 4-chloroaniline: a computational study. Physica E 76, 6–11.
M. Samadizadeh, A. A. Peyghan, and S. F. Rastegar (2015). Sensing behavior of BN nanosheet toward nitrous oxide: A DFT study. Chin. Chem. Lett. 26, 1042–1045.
H. Wang, T. Maiyalagan, and X. Wang (2012). Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2, 781–794.
Y. Zhao and D. G. Truhlar (2006). A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 125, 194101.
Y. Zhao and D. G. Truhlar (2008). The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241.
Y. Zhao and D. G. Truhlar (2006). Density functional for spectroscopy: no long-range self-interaction error, good performance for Rydberg and charge-transfer states, and better performance on average than B3LYP for ground states. J. Phys. Chem. A 110, 13126–13130.
E. Vessally, S. Soleimani-Amiri, A. Hosseinian, L. Edjlali, and A. Bekhradnia (2017). The Hartree-Fock exchange effect on the CO adsorption by the boron nitride nanocage. Physica E 87, 308–311.
Acknowledgements
The authors wish to acknowledge from Mazandaran University of Medical Sciences and Payame Noor University for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Javarsineh, S.A., Vessally, E., Bekhradnia, A. et al. A Computational Study on the Purinethol Drug Adsorption on the AlN Nanocone and Nanocluster. J Clust Sci 29, 767–775 (2018). https://doi.org/10.1007/s10876-018-1381-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-018-1381-7