Abstract
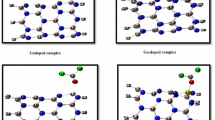
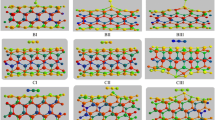
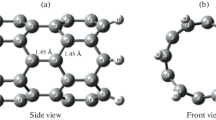
Adsorption of the HCN molecule is very important in environment and industrial applications. The BeONT may be good candidate for HCN capture because of large surface. Unfortunately, BeONT shows limited HCN detection. Therefore, we investigate the possibility of HCN adsorption on Ca and Mg-doped BeONT by density functional theory calculations. It was found that HCN adsorption on doped nanotube has relatively higher adsorption energy as compared with the perfect one. Furthermore, there exists a strong adsorption between HCN molecule and doped nanotubes, which exhibited more active interaction and larger net charge transfer than that of pristine nanotube. As well as, calculated geometrical parameters and electronic properties for studied systems indicate that the Ca-doped BeONT and Mg-doped BeONT present high sensitivity to HCN, compared with the pristine BeONT. Theoretical results reveal that the adsorption of the HCN on the doped nanotube is influenced on the electronic conductance of the doped-BeONT. Therefore, Ca and Mg-doped nanotube can be considered as promising sensor for detecting HCN molecule. According to NBO analysis, electron flow is spontaneous from doped nanotube to HCN molecule.




Similar content being viewed by others
References
T. L. Blank, M. V. Roloff, R. D. Short, S. M. Schuengel, and W. E. Ribelin (2002). Toxicol. Lett. 18, (Suppl. 1), 136.
J. Kong, N. R. Franklin, C. Zhou, M. G. Chapline, S. Peng, K. Cho, and H. Dai (2000). Science 287, 622–625.
P. G. Collins, K. Bradley, M. Ishigami, and A. Zettl (2000). Science 287, 1801–1804.
H. Raissi and F. Mollania (2014). Eur. J. Pharm. Sci. 56, 37–54.
S. Duman, A. Sütlü, S. Bagci, H. M. Tütüncü, and G. P. Srivastava (2009). J. Appl. Phys. 105, 033719-1–033719-8.
P. B. Sorokin, A. S. Fedorov, and L. A. Chernozatonskii (2006). Phys. Solid State 48, 398–401.
B. Baumeier, P. Krüger, and J. Pollmann (2007). Phys. Rev. B 76, 085407.1–085407.8.
M. A. Gorbunova, I. R. Shen, Y. N. Makurin, V. V. Ivanovskaya, and A. L. Ivanovskii (2008). Physica E 41, 164.
A. D. Becke (1993). J. Chem. Phys. 98, 5648–5652.
C. Lee, W. Yang, and R. G. Parr (1988). Phys. Rev. B 37, 785–789.
S. B. Boys and F. Bernardi (1970). Mol. Phys. 19, 553–566.
T. A. Koopmans (1934). Physica 1, 104–113.
R. G. Pearson (1985). J. Am. Chem. Soc. 107, 6801–6806.
R. G. Parr and P. K. Chattaraj (1991). J. Am. Chem. Soc. 113, 1854–1855.
A. E. Reed, L. A. Curtiss, and F. Weinhold (1988). Chem. Rev. 88, 899–926.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. J. A. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03, Revision C. 02, Gaussian, Inc.: Wallingford, CT (2004).
R. F. W. Bader, A. Streitwieser, A. Neuhaus, K. F. Laidig, and P. Speers (1996). J. Am. Chem. Soc. 118, 4959–4965.
F. Biegler- König and J. Schönbohm (2002). J. Comput. Chem. 23, 1489–1494.
A. W. Ehlers, E. J. Baerends, and K. Lammertsma (2002). J. Am. Chem. Soc. 124, 2831–2838.
K. K. Pandey and G. Frenking (2004). Eur. J. Inorg. Chem. 2004, 4388–4395.
M. K. Cyrański, T. M. Krygowski, A. R. Katritzky, and P. V. R. Schleyer (2002). J. Org. Chem. 67, 1333–1338.
P. Pyykkö (2001). Mol. Phys. 99, 1617–1629.
M. Marvi, H. Raissi, and H. Ghiassi (2016). Struct. Chem.. doi:10.1007/s11224-015-0585-9.
M. Moradi, M. Noei, and A. Ahmadi Peyghan (2013). Mol. Phys. 111, (21), 3320–3326.
A. Ahmadi Peyghan, S. F. Rastegar, and N. L. Hadipour (2014). Phys. Lett. A. 378, (30), 2184–2190.
N. L. Hadipour, A. Ahmadi Peyghan, H. Soleymanabadi (2015). J. Phys. Chem. C. 9, 6398–6404.
M. Eslami, V. Vahabi, and A. Ahmadi Peyghan (2016). Physica E. 76, 6–11.
M. Samadizadeh, A. Ahmadi Peyghan, and S. F. Rastegar (2015). Chin. Chem. Lett. 26, (8), 1042–1045.
Acknowledgements
We perform some calculations with Saffron HPC cluster.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghiassi, H., Raissi, H. & Marvi, M. Boosting BeONT Reactivity with HCN by Calcium and Magnesium Doping: A DFT Investigation of Electronic Structure, AIM, NMR, NQR and NBO Analysis. J Clust Sci 29, 101–110 (2018). https://doi.org/10.1007/s10876-017-1310-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1310-1