Abstract
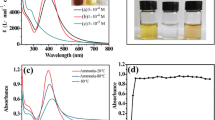
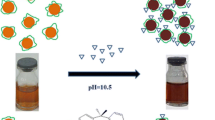
In the present study, a simple and eco-friendly method was developed for preparation of stable silver chloride nanoparticles by aqueous extract of Sargassum boveanum alga collected from the Persian Gulf. By increasing the pH, the as-prepared silver chloride nanoparticles were transformed to the silver nanoparticles. The green synthesized silver chloride nanoparticles were developed as a colorimetric assay for determination of monosaccharides. The color of solution changed from bright yellow to dark brown gradually when an increase of monosaccharides concentration occurred and a new absorption band appeared at higher wavelengths which indicated the aggregation of silver nanoparticles. In fact, the production rate of silver nanoparticles increased in the presence of monosaccharides at alkaline condition. Moreover, the enlargement and aggregation of silver nanoparticles occurred. The absorbance at 600 nm was found to be linearly dependent on the glucose, fructose and galactose concentrations in the range of 5–900, 1–500 and 1–300 μM with a limit of detection of 1, 0.5 and 0.5 μM respectively. Finally, the proposed green synthesized nanoparticles showed high selectivity toward monosaccharides determination in alkaline solution.












Similar content being viewed by others
References
D. Li, A. Wieckowska, and I. Willner (2008). Angew. Chem. Int. Ed. 47, 3927.
D. Vilela, M. C. González, and A. Escarpa (2012). Anal. Chim. Acta 751, 24.
R. J. Stokes, A. MacAskill, P. J. Lundahl, W. E. Smith, K. Faulds, and D. Graham (2007). Small 9, 1593.
V. Pitthard and P. Finch (2001). Chromatographia 53, S317.
J. D. Olivera, M. Gaborieaub, E. F. Hilderc, and P. Castignolles (2013). J. Chromatogr. A 1291, 179.
J.-H. Xie, M.-Y. Shen, S.-P. Nie, X. Liu, H. Zhang, and M.-Y. Xie (2013). Carbohydr. Polym. 98, 976.
W. S. York, S. Hantus, P. Albersheim, and A. G. Darvill (1997). Carbohydr. Res. 300, 199.
N. DiCesare and J. R. Lakowicz (2001). Chem. Commun. 2022.
N. DiCesare and J. R. Lakowicz (2002). Tetrahedron Lett. 43, 2615.
R. Badugu, J. R. Lakowicz, and C. D. Geddes (2006). Dyes Pigments 68, 159.
M. He, R. J. Johnson, J. O. Escobedo, P. A. Beck, B. J. Melancon, W. D. Treleaven, R. M. Strongin, P. T. Lewis, K. K. Kim, N. N. St Luce, A. A. Mrse, C. J. Davis, and F. R. Fronczek (2002). J. Am. Chem. Soc. 124, 5000.
L. Saa, M. Coronado-Puchau, V. Pavlov, and L. M. Liz-Marzán (2014). Nanoscale 6, 7405.
L. Fruk and C. M. Niemeyer (2005). Angew. Chem. Int. Ed. 44, 2603.
C. Xu and Z. Zhang (2001). Anal. Sci. 17, 1449.
X.-M. Huang, M. Zhu, L.-Y. Mao, and H.-X. Shen (1997). Anal. Sci. 13, 145.
B. Tang, G.-Y. Zhang, Y. Liu, and F. Han (2002). Anal. Chim. Acta 459, 83.
H. Wei and E. Wang (2008). Anal. Chem. 80, 2250.
H. Jiang, Z. Chen, H. Cao, and Y. Huang (2012). Analyst 137, 5560.
L. Su, J. Feng, X. M. Zhou, C. L. Ren, H. H. Li, and X. G. Chen (2012). Anal. Chem. 84, 5753.
A. K. Dutta, S. Das, S. Samanta, P. K. Samanta, B. Adhikary, and P. Biswas (2013). Talanta 107, 361.
Q. Liu, Q. Jia, R. Zhua, Q. Shao, D. Wang, P. Cui, and J. Ge (2014). Mater. Sci. Eng. C 42, 177.
W. Shi, X. Zhang, S. He, and Y. Huang (2011). Chem. Commun. 47, 10785.
F. Qiao, L. Chen, X. Li, L. Li, and S. Ai (2014). Sens. Actuator B 193, 255.
W. Chen, J. Chen, Y.-B. Feng, L. Hong, Q.-Y. Chen, L.-F. Wu, X.-H. Lina, and X.-H. Xia (2012). Analyst 137, 1706.
Y. L. Liu, X. J. Zhao, X. X. Yang, and Y. F. Li (2013). Analyst 138, 4526.
R. Cui, Z. Han, and J.-J. Zhu (2011). Chem. Eur. J. 17, 9377.
Y. Song, K. Qu, C. Zhao, J. Ren, and X. Qu (2010). Adv. Mater. 22, 2206.
W. Shi, Q. Wang, Y. Long, Z. Cheng, S. Chen, H. Zheng, and Y. Huang (2011). Chem. Commun. 47, 6695.
A. Pandya, P. G. Sutariya, and S. K. Menon (2013). Analyst 138, 2483.
K. Cao, X. Jiang, S. Yan, L. Zhang, and W. Wu (2014). Biosens. Bioelectron. 52, 188.
Q. Zhao, S. Chen, H. Huang, L. Zhang, L. Wang, F. Liu, J. Chen, Y. Zeng, and P. K. Chu (2014). Analyst 139, 1498.
J. Yuana, W. Guoa, J. Yinb, and E. Wang (2009). Talanta 77, 1858.
N. Asmathunisha and K. Kathiresan (2013). Colloids Surf. B 103, 283.
G. Singaravelu, J. S. Arockiamary, V. Ganesh Kumar, and K. Govindaraju (2007). Colloids Surf. B 57, 97.
Y. N. Mata, E. Torres, M. L. Blázquez, A. Ballester, F. González, and J. A. Mũnoz (2009). J. Hazard. Mater. 166, 612.
C. M. Ramakritinan, E. Kaarunya, S. Shankar, and A. K. Kumaraguru (2013). Solid State Phenom. 201, 211.
M. Vivek, P. S. Kumar, S. Steffi, and S. Sudha (2011). Avicenna J. Med Biotechnol. 3, 143.
T. Stalin Dhas, V. Ganesh Kumar, V. Karthick, K. Jini Angel, and K. Govindaraju (2014). Spectrochim. Acta A 120, 416.
S. Momeni and I. Nabipour (2015). Appl. Biochem. Biotechnol. 176, 1937.
P. Atkins and J. de Paula Physical Chemistry, 8th ed (W.H. Freeman, New York, 2006).
C. An, S. Peng, and Y. Sun (2010). Adv. Mater. 22, 2570.
H. Xu, H. Li, J. Xia, S. Yin, Z. Luo, L. Liu, and L. Xu (2011). ACS Appl. Mater. Interfaces 3, 22.
G. Wang, T. Nishio, M. Sato, A. Ishikawa, K. Nambara, K. Nagakawa, Y. Matsuo, K. Niikura, and K. Ijiro (2011). Chem. Commun. 47, 9426.
G. Wang, H. Mitomo, Y. Matsuo, K. Niikura, M. Maeda, and K. Ijiro (2015). J. Colloid Interface Sci. 452, 224.
J. T. Huang, X. X. Yang, Q. L. Zenga, and J. Wang (2013). Analyst 138, 5296.
S. Nishimura, D. Mott, A. Takagaki, S. Maenosono, and K. Ebitani (2011). Phys. Chem. Chem. Phys. 13, 9335.
M. Darroudi, M. B. Ahmad, A. H. Abdullah, N. A. Ibrahim, and K. Shameli (2010). Int. J. Mol. Sci. 11, 3898.
Acknowledgements
This project was partly supported by Iran National Science Foundation (Research Chair Award No. 95/INSF/44913).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Momeni, S., Nabipour, I., Karimi, S. et al. New Colorimetric Detection of Monosaccharides Based on Transformation of Silver Chloride Nanoparticles to Silver Nanoparticles Synthesized by Sargassum Alga. J Clust Sci 28, 2205–2221 (2017). https://doi.org/10.1007/s10876-017-1220-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1220-2