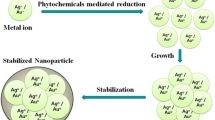
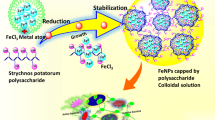
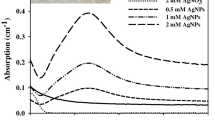
Abstract
Green chemistry is the torch bearing field of sustainable research where without use of any toxic chemicals, environment-friendly metal nanoparticles are produced. Advantages of green nanoparticle synthesis over chemical-based synthesis are its nearly zero toxicity with wider applications. As the multidrug resistant species begin to emerge, green synthesized nanoparticles have been arisen as a potent alternative of antimicrobials along with various other applications in diverse fields. The main hindrances behind green synthesis are choice of material and its availability. Because of cheaper cost, wide availability, enhanced effectivity and fewer side effects, polysaccharides have successfully replaced the position of chemical reducing agents in nanoparticle synthesis. Our present review focuses on preparation and applications of polysaccharide based metal nanoparticles; a state-of-the-art research with special emphasis on green synthesized silver nanoparticles as a potent source of emerging antimicrobial.

(information source: Relecura)

(information source: Relecura)
Similar content being viewed by others
References
M. C. Daniel and D. Astruc (2004). J. Chem. Rev. 104, 293–346.
A. Krolikowska, A. Kudelski, A. Michota, and J. Bukowska (2003). Surf. Sci. 532, 227–232.
V. P. Zharov, J. W. Kim, D. T. Curiel, and M. Everts (2005). Nanotechnol. Biol. Med. 1, 326–345.
A. Kumar, S. Mandal, P. R. Selvakannan, R. Parischa, A. B. Mandale, and M. Sastry (2003). Langmuir. 19, 6277–6282.
K. Bogunia-Kubik and M. Sugisaka (2002). BioSystems. 65, 123–138.
M. Shah, D. Fawcett, S. Sharma, S. K. Tripathy, and G. E. J. Poinern (2015). Materials 8, 7278–7308.
M. Esparza-Soto and P. Westerhoff (2003). Water Res. 37, 2301–2310.
J. C. Liu, G. Qin, P. Raveendran, and P. Ikushimax (2006). Chem. Eur. J. 12, 2131–2138.
K. Balantrapu and D. V. Goia (2009). J. Mater. Res. 24, 2828–2836.
M. A. Albrecht, C. W. Evans, and C. L. Raston (2006). Green Chem. 8, 417–432.
T. Sun and K. Seff (1994). Chem. Rev. 94, 857–870.
D. J. Xiong, M. L. Chen, and H. Li (2008) Chem. Commun. 7, 880–882.
N. Duran, P. D. Marcato, O. L. Alves, G. I. H. De Souza, and E. J. Esposito (2005). J. Nanobiotechnology. 3, 8.
S. Basu, S. Jana, S. Pande, and T. J. Pal (2008). J. Colloid Interface Sci. 321, 288–293.
I. Brigger, C. Dubernet, and P. Couvreur (2004). P. Adv. Drug. Deliv. Rev. 54, 631–651.
M. G. Guzman, J. Dille, and S. Godet (2008). World Acad. Sci. Eng. Technolo. 43, 357–364.
Z. Zhu, L. Kai, and Y. Wang (2006). Mater. Chem. Phys. 96, 447–453.
I. Sondi and B. J. Salopek-Sondi (2004). J. Colloid Interface Sci. 275, 177–182.
D. Yu and V. W. Yam (2004). J. Am. Chem. Soc. 126, 13200–13201.
M. Harada, Y. Inada, and M. J. Nomura (2009). J. Colloid Interface Sci. 337, 427–438.
S. T. Dubas and V. Pimpan (2008). Talanta 76, 29–33.
A. Taleb, C. Petit, and M. P. Pileni (1997). Chem. Mater. 9, 950–959.
A. Henglein (2001). Langmuir 17, 2329–2333.
K. Esumi, T. Tano, K. Torigoe, and K. Meguro (1990). Chem. Mater. 2, 564–567.
J. J. Zhu, S. W. Liu, O. Palchik, Y. Koltypin, and A. Gedanken (2000). Langmuir 16, 6396–6399.
V. K. Sharma, R. A. Yngard, and Y. Lin (2009). Ads. Colloid Interface Sci. 145, 83–96.
J. Xie, J. Y. Lee, D. I. C. Wang, and Y. P. Ting (2007). ACS Nano. 1, 429–439.
J. I. Hussain, S. Kumar, A. A. Hashmi, and Z. Khan (2011). Adv. Mat. Lett. 2, 188–194.
Y. Park, Y. N. Hong, A. Weyers, Y. S. Kim, and R. J. Linhardt (2011). IET Nanobiotechnol. 5, 69–78.
D. A. Geraldo, P. Needham, N. Chandia, R. Arratia-Perez, G. C. Mora, and N. A. Villagra (2016). Biointerface Res. Appl. Chem. 6, 1263–1271.
S. Singh, A. S. Vidyarthi, V. K. Nigam, and A. Dev (2014). Artif. Cells Nanomed. Biotechnol. 42, 6–12.
P. Kanmani and S. T. Lim (2013). Process Biochem. 48, 1099–1106.
R. Selvakumar, S. Aravindh, A. M. Ashok, and Y. L. Balachandran (2014). J. Exp. Nanosci. 9, 1075–1087.
G. Sathiyanarayanan, G. S. Kiran, and J. Selvin (2013). Colloids Surf., B. 102, 13–20.
V. Venkatpurwar and V. Pokharkar (2011). Mater. Lett. 65, 999–1002.
A. Travan, C. Pelillo, I. Donati, E. Marsich, M. Benincasa, T. Scarpa, S. Semeraro, G. Turco, R. Gennaro, and S. Paoletti (2009). Biomacromolecules 10, 1429–1435.
A. J. Kora, S. R. Beedu, and A. Jayaraman (2012). Org. Med. Chem. Lett. 2, 17.
G. Li, Y. Li, Z. Wang, and H. Liu (2017). Mater. Chem. Phys. 187, 133–140.
S. K. Srikar, D. D. Giri, D. B. Pal, P. K. Mishra, and S. N. Upadhyay (2016). Green and Sustainable Chemistry 6, 34.
B. Le Ouay and F. Stellacci (2015). Nano Today 10, 339–354.
S. Pal, Y. K. Tak, and J. M. Song (2007). Appl. Environ. Microbiol. 73, 1712–1720.
A. Nanda and C. M. Raghavan (2014). Int. J. Chem. Tech. Res. 6, 2914–2919.
M. Rai, A. Yadav, and A. Gade (2009). Biotechnol. Adv. 27, 76–83.
F. Baldi, S. Daniele, M. Gallo, S. Paganelli, D. Battistel, O. Piccolo, C. Faleri, A. M. Puglia, and G. Gallo (2016). BioMetals 29, 321–331.
H. M. El-Rafie, M. H. El-Rafie, and M. K. Zahran (2013). Carbohydr. Polym. 96, 403–410.
K. Murugan, A. Jaganathan, U. Suresh, R. Rajaganesh, S. Jayasanthini, A. Higuchi, S. Kumar, and G. Benelli (2017). J. Clust. Sci. 28, 529–550.
G. Benelli, P. Roman, M. Filippo, P. Riccardo, and Marcello Nicoletti (2017). J. Clust. Sci. 28, 3–10.
G. Benelli and C. M. Lukehart (2017). J. Clust. Sci. 28, 1–2.
K. Murugan, A. Jaganathan, D. Devakumar, S. Udaiyan, R. Rajapandian, C. Balamurugan, J. Subramaniam, M. Paulpandi, C. Vadivalagan, P. Amuthavalli, L. Wang, J. Hwang, H. Wei, M. S. Alsalhi, S. Devanesan, S. Kumar, K. Pugazhendy, A. Higuchi, M. Nicoletti, and G. Benelli (2016). Ecotoxicol. Environ. Saf. 132, 318–328.
R. Patel and S. Suresh (2006). J. Hazard. Mater. 137, 1729e1741.
A. Safavi and S. Momeni (2012). J. Hazard. Mater. 201, 125e131.
W. Salem, D. R. Leitner, F. G. Zingl, G. Schratter, R. Prassl, W. Goessler, J. Reidl, and S. Schild (2015). Int. J. Med. Microbiol. 305, 85–95.
G. Benelli (2017). J. Clust. Sci. 28, 11–14.
A. J. Kora, R. B. Sashidhar, and J. Arunachalam (2010). Carbohydr. Polym. 82, 670–679.
A. Mehta, C. Sidhu, A. K. Pinnaka, and A. R. Choudhury (2014). PloS one 9, e98798.
Acknowledgements
Authors are thankful to UGC-Center of Advanced Study, Department of Botany, The University of Burdwan for pursuing research activities. Aparna Banerjee is also thankful to JRF (State Funded) for the financial assistance [Fc (Sc.)/RS/SF/BOT./2014-15/103 (3)].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banerjee, A., Halder, U. & Bandopadhyay, R. Preparations and Applications of Polysaccharide Based Green Synthesized Metal Nanoparticles: A State-of-the-Art. J Clust Sci 28, 1803–1813 (2017). https://doi.org/10.1007/s10876-017-1219-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1219-8