Abstract
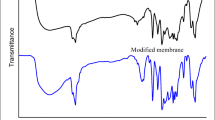
Novel cation-exchange nanocomposite membranes was fabricated by in-situ formation of zinc oxide nanoparticles in a blend containing sulfonated polyvinylchloride and sulfonated poly(2,6-dimethyl-1,4-phenylene oxide) by a simple chemical method. Also, some nanocomposite samples were prepared by incorporation of previously synthesized ZnO nanoparticles into the casting solution (ex-situ method). The prepared nanocomposite samples and nanoparticles were characterized by several techniques including water contact angle measurements, scanning electron microscopy, Fourier transform infrared spectroscopy and X-ray diffraction. The water contact angle measurements confirmed the increased hydrophilicity of the nanocomposite membranes. The SEM surface microphotographs indicated that ZnO nanoparticles were uniformly dispersed throughout the polymeric matrix. The influence of additive concentration on electrochemical and physicochemical properties of prepared ion-exchange nanocomposite membranes was studied. Various investigations revealed that the in-situ preparation of ZnO nanoparticles in the membrane structure had a considerable effect on the membrane efficiency and could improve the transport properties.












Similar content being viewed by others
Abbreviations
- A:
-
Membrane surface area (m2)
- a:
-
Milli-equivalent of ion-exchange groups in membrane (meq)
- a1, a2 :
-
Ions electrolytic activities
- C.E:
-
Current efficiency
- Cmean :
-
Mean concentration of electrolytes (M)
- d:
-
Membrane thickness (m)
- Em :
-
Membrane potential (mV)
- F:
-
Faraday constant
- FT-IR:
-
Fourier transform infrared spectroscopy
- I:
-
Current intensity (A)
- IEC:
-
Ion-exchange capacity
- IEMS:
-
Ion-exchange membranes
- n, Zi :
-
Electrovalence of ion
- Ps :
-
Membrane ionic permselectivity
- R:
-
Universal gases constant (J mol−1 K−1)
- r:
-
Specific electrical resistance (Ω cm2)
- R1 and R2 :
-
Electrical resistance (Ω)
- Rm :
-
Membrane resistance (Ω)
- SEM:
-
Scanning electron microscope
- SPPO:
-
Sulfonated poly(2,6-dimethyl-1,4-phenylene oxide)
- SPVC:
-
Sulfonated polyvinylchloride
- T:
-
Temperature (K)
- t:
-
Time (min)
- THF:
-
Tetrahydrofuran
- t mi ; t0 :
-
Transport number of counter ions in membrane phase; in solution
- Y:
-
Concentration of fixed charge on the membrane surface
- Δn:
-
Number of transported moles through membrane
References
X. Zuo, S. Yu, X. Xu, J. Xu, R. Bao, and X. Yan (2009). J. Membr. Sci. 340, 206.
L. J. Banasiak, B. Van der Bruggen, and A. I. Schafer (2011). Chem. Eng. J. 166, 233.
F. Karimi, S. N. Ashrafizadeh, and F. Mohammadi (2012). Chem. Eng. J. 183, 402.
H. J. Lee, M. K. Hong, and S. H. Moon (2012). Desalination 284, 221.
J. Luo, C. Wu, T. Xu, and Y. Wu (2011). J. Membr. Sci. 366, 1.
M. C. Martí-Calatayud, D. C. Buzzi, M. García-Gabaldón, A. M. Bernardes, J. A. S. Tenório, and V. Pérez-Herranz (2014). J. Membr. Sci. 466, 45.
S. H. Moon and S. H. Yun (2014). Curr. Opin. Chem. Eng. 4, 25.
P. V. Vyas, B. G. Shah, G. S. Trivedi, P. Ray, S. K. Adhikary, and R. Rangarajan (2001). J. Membr. Sci. 187, 39.
P. V. Vyas, B. G. Shah, G. S. Trivedi, P. Ray, S. K. Adhikary, and R. Rangarajan (2000). React. Funct. Polym. 44, 101.
C. Vogel and J. Meier-Haack (2014). Desalination 342, 156.
K. A. Mauritz (1998). Mater. Sci. Eng. C 6, 121.
M. L. Sforca, I. V. P. Yoshida, and S. P. Nunes (1999). J. Membr. Sci. 159, 197.
R. K. Nagarale, G. S. Gohil, V. K. Shahi, G. S. Trivedi, and R. Rangarajan (2004). J. Colloid Interface Sci. 277, 162.
M. M. A. Khan (2012). J. Appl. Polym. Sci. 124, 338.
P. Xu, G. M. Zeng, D. L. Huang, C. L. Feng, S. Hu, M. H. Zhao, C. Lai, Z. Wei, C. Huang, G. X. Xie, and Z. F. Liu (2012). Sci. Total Environ. 424, 1.
L. Y. Ng, A. W. Mohammad, C. P. Leo, and N. Hilal (2013). Desalination 308, 15.
A. R. Khodabakhshi, S. S. Madaeni, T. W. Xu, L. Wu, C. Wu, C. Li, W. Na, S. A. Zolanvari, A. Babayi, J. Ghasemi, S. M. Hosseini, and A. Khaledi (2012). Sep. Purif. Technol. 90, 10.
T. W. Xu, W. H. Yang, and B. L. He (2002). Chin. J. Polym. Sci. 20, 53.
T. W. Xu, D. Wu, and L. Wu (2008). Prog. Polym. Sci. 33, 894.
H. Yu and T. W. Xu (2006). J. Appl. Polym. Sci. 100, 2238.
D. Wu, L. Wu, J. J. Woo, S. H. Yun, S. J. Seo, T. W. Xu, and S. H. Moon (2010). J. Membr. Sci. 348, 167.
X. Zhang, Y. Chen, A. H. Konsowa, X. Zhu, and J. C. Crittenden (2009). Sep. Purif. Technol. 70, 71.
L. Xu and H. K. Lee (2009). J. Chromatogr. A 1216, 6549.
M. Yousefi, E. Noori, D. Ghanbari, M. Salavati-Niasari, and T. Gholami (2014). J. Clust. Sci. 25, 397.
R. Li, J. Pei, and C. Sun (2015). Constr. Build. Mater. 98, 656.
P. G. Freire, R. H. O. Montes, F. C. Romeiro, S. C. S. Lemos, R. C. Lima, E. M. Richter, and R. A. A. Munoz (2016). Sens. Actuators B 223, 557.
H. Esmailzadeh, P. Sangpourb, F. Shahraza, J. Hejazi, and R. Khaksar (2016). Mater. Sci. Eng. C 58, 1058.
K. Girigoswami, M. Viswanathan, R. Murugesan, and A. Girigoswami (2015). Mater. Sci. Eng. C 56, 501.
J. Archana, M. Navaneethan, and Y. Hayakawa (2016). Scr. Mater. 113, 163.
A. Susanna, L. Armelao, E. Callone, S. Dirè, M. D’Arienzo, B. Di Credico, L. Giannini, T. Hanel, F. Morazzoni, and R. Scotti (2015). Chem. Eng. J. 275, 245.
T. W. Xu, W. H. Yang, and B. L. He (2001). Chem. Eng. Sci. 56, 5343.
F. Heidary, A. Nemati Kharat, and A. R. Khodabakhshi (2016). J. Clust. Sci. 27, 193.
H. Strathmann Membrane Separations Technology: Principles and Applications (Elsevier, New York, 1995).
S. Bahadorikhalili, L. Ma’mani, H. Mahdavi, and A. Shafiee (2015). RSC Adv. 5, 71297.
X. Li, Z. Wang, H. Lu, C. Zhao, H. Na, and C. Zhao (2005). J. Membr. Sci. 254, 147.
R. K. Nagarale, V. K. Shahi, and R. Rangarajan (2005). J. Membr. Sci. 248, 37.
G. S. Gohil, V. V. Binsu, and V. K. Shahi (2006). J. Membr. Sci. 280, 210.
J. Schauer, V. Kudela, K. Richau, and R. Mohr (2006). Desalination 198, 256.
L. Lebrun, E. Da Silva, G. Pourcelly, and M. Métayer (2003). J. Membr. Sci. 227, 95.
D. R. Lide CRC Handbook of Chemistry and Physics (CRC Press, Boca Raton, 2010).
A. R. Khodabakhshi, S. S. Madaeni, and S. M. Hosseini (2011). Sep. Purif. Technol. 77, 220.
B. Heidari, M. Ansari, A. Hoseinabadi, H. Jiriaee, and F. Heidary (2016). J. Mater. Sci. Mater. Electron. doi:10.1007/s10854-016-5625-8.
Y. Tanaka Ion Exchange Membranes: Fundamentals and Applications, Membrane Science and Technology Series (Elsevier, Amsterdam, 2007).
R. K. Nagarale, G. S. Gohil, and V. K. Shahi (2006). Adv. Colloid Interface Sci. 119, 97.
A. Shau, P. De, and P. Ray (2016). Int. J. Hydrog. Energy 41, 10292.
M. Y. Kariduraganavar, R. K. Nagarale, A. A. Kittur, and S. S. Kulkarni (2006). Desalination 197, 225.
W. Wan, T. J. Pepping, T. Banerji, S. Chaudhari, and D. E. Giammar (2011). Water Res. 45, 384.
V. K. Shahi, R. Prakash, G. Ramachandraiah, R. Rangarajan, and D. Vasudevan (1999). J. Colloid Interface Sci. 216, 179.
R. K. Nagarale, V. K. Shahi, S. K. Thampy, and R. Rangarajan (2004). React. Funct. Polym. 61, 131.
F. Heidary, A. R. Khodabakhshi, and A. Nemati Kharat (2016). Korean J. Chem. Eng. 33, 1380.
S. A. Patil and T. M. Aminabhavi (2006). J. Membr. Sci. 281, 95.
R. Shahabadi, M. Abdollahi, and A. Sharif (2015). Int. J. Hydrog. Energy 40, 3749.
Acknowledgements
The authors are thankful to Laboratory of Functional Membranes (University of Science and Technology of China) for providing SPPO.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Heidary, F., Khodabakhshi, A.R. & Ghanbari, D. A Novel Sulfonated Poly Phenylene Oxide-Poly Vinylchloride/ZnO Cation-Exchange Membrane Applicable in Refining of Saline Liquids. J Clust Sci 28, 1489–1507 (2017). https://doi.org/10.1007/s10876-017-1156-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1156-6