Abstract
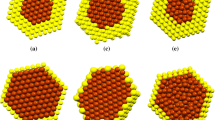
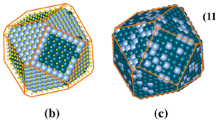
The structures and thermal properties of Ag–Pt–Ni ternary nanoclusters varying with different compositions and sizes are studied by Monte Carlo and molecular dynamics simulations. It can be found that silver atoms tend to occupy the surface and platinum atoms favor the subsurface occupation, whereas the inner is occupied by nickel atoms due to the different surface energies and lattice parameters. In addition, there is a non-monotonous relationship between the melting points and compositions of Ag–Pt–Ni ternary nanoclusters according to molecular dynamics simulations. In addition, a linear decrease in melting point with \(N^{ - 1/3}\) is found for both monometallic and trimetallic clusters. This behavior is consistent with Pawlow’s law.









Similar content being viewed by others
References
S. S. Fenton, V. Ramani, and J. M. Fenton (2006). Electrochem. Soc. Interface 15, 37.
J. Carton, V. Lawlor, A. Olabi, C. Hochenauer, and G. Zauner (2012). Energy 39, 63.
D. C. Higgins and Z. Chen (2013). Can. J. Chem. Eng. 91, 1881.
F. Jaouen, E. Proietti, M. Lefèvre, R. Chenitz, J.-P. Dodelet, G. Wu, H. T. Chung, C. M. Johnston, and P. Zelenay (2011). Energy Environ. Sci. 4, 114.
A. A. Gewirth and M. S. Thorum (2010). Inorg. Chem. 49, 3557.
Z. Peng, J. Wu, and H. Yang (2009). Chem. Mater. 22, 1098.
M. K. Carpenter, T. E. Moylan, R. S. Kukreja, M. H. Atwan, and M. M. Tessema (2012). J. Am. Chem. Soc. 134, 8535.
X. Song and D. Zhang (2014). Energy 70, 223.
Z. Lei, Z. Ding, S. Ming, C. Yungui, and W. Xiaofei (2014). Rare Metal Mat. Eng. 43, 2507.
B. Fang, J. Luo, P. N. Njoki, R. Loukrakpam, B. Wanjala, J. Hong, J. Yin, X. Hu, J. Last, and C. J. Zhong (2010). Electrochim. Acta 55, 8230.
J. N. Tiwari, R. N. Tiwari, G. Singh, and K. S. Kim (2013). Nano Energy 2, 553.
B. N. Wanjala, B. Fang, J. Luo, Y. Chen, J. Yin, M. H. Engelhard, R. Loukrakpam, and C. J. Zhong (2011). J. Am. Chem. Soc. 133, 12714.
P. Mani, R. Srivastava, and P. Strasser (2011). J. Power Sources 196, 666.
D. Wu and D. Cheng (2015). Electrochimica Acta 180, 316.
D. Cheng, X. Liu, D. Cao, W. Wang, and S. Huang (2007). Nanotechnology 18, 475702.
R. Subbaraman and S. K. Sankaranarayanan (2011). Surf. Sci. 605, 1595.
S. Khanal, N. Bhattarai, J. J. Velazquez-Salazar, D. Bahena, G. Soldano, A. Ponce, M. M. Mariscal, S. Mejia-Rosales, and M. Jose-Yacaman (2013). Nanoscale 5, 12456.
Z. Zhao, M. Li, D. Cheng, and J. Zhu (2014). Chem. Phys. 441, 152.
F. Cleri and V. Rosato (1993). Phys. Rev. B 48, 22.
D. Cheng and D. Cao (2008). Eur. Phys. J. B 66, 17.
L. O. Paz-Borbón, R. L. Johnston, G. Barcaro, and A. Fortunelli (2008). J. Chem. Phys. 128, 134517.
Z. Kuntová, G. Rossi, and R. Ferrando (2008). Phys. Rev. B 77, 205431.
F. Baletto and R. Ferrando (2005). Rev. Mod. Phys. 77, 371.
G. Rossi, A. Rapallo, C. Mottet, A. Fortunelli, F. Baletto, and R. Ferrando (2004). Phys. Rev. Lett. 93, 105503.
R. Ferrando, J. Jellinek, and R. L. Johnston (2008). Chem. Rev. 108, 845.
L. Wang and D. D. Johnson (2009). J. Am. Chem. Soc. 131, 14023.
G. G. Guisbiers, R. N. Mendoza-Cruz, L. Bazán-Díaz, J. J. Velázquez-Salazar, R. Mendoza-Perez, J. A. Robledo-Torres, J. L. Rodriguez-Lopez, J. M. Montejano-Carrizales, R. L. Whetten, and M. José-Yacamán (2015). ACS nano 10, 188.
F. Aqra and A. Ayyad (2011). Appl. Surf. Sci. 257, 6372.
C. Kittel and D. F. Holcomb (1967). Am. J. Phys. 35, 547.
S. K. Sankaranarayanan, V. R. Bhethanabotla, and B. Joseph (2005). Phys. Rev. B 71, 195415.
P. Puri and V. Yang (2007). J. Phys. Chem. C 111, 11776.
K. Fukui, B. G. Sumpter, M. D. Barnes, and D. W. Noid (1999). Polym. J. 31, 664.
Y. Qi, T. Çağın, Y. Kimura, and W. A. Goddard III (1999). Phys. Rev. B 59, 3527.
P. J. Steinhardt, D. R. Nelson, and M. Ronchetti (1983). Phys. Rev. B 28, 784.
Y. Wang, S. Teitel, and C. Dellago (2004). Chem. Phys. Lett. 394, 257.
P. Pawlow (1909). Z. phys. Chem 65, 545.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (21576008, 91334203), BUCT Fund for Disciplines Construction and Development (Project No. XK1501), Fundamental Research Funds for the Central Universities (Project No. buctrc201530 and PT1613-01), and “Chemical Grid Project” of BUCT.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wei, C., Zhao, Z., Fisher, A. et al. Theoretical Study on the Structures and Thermal Properties of Ag–Pt–Ni Trimetallic Clusters. J Clust Sci 27, 1849–1861 (2016). https://doi.org/10.1007/s10876-016-1068-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1068-x