Abstract
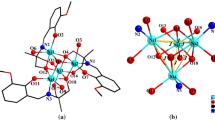
Two tetranuclear complexes, [M(H3L)]4·X (1, M = Cu, X = 4,4′-dpdo; 2, M = Ni, X = DMF, H5L = 2-[(3,5-dibromo-2-hydroxybenzyl) amino]-2-(hydroxymethyl)propane-1,3-diol, 4,4′-dpdo is 4,4′-bipyridine-N,N′-dioxide, DMF = N,N′-dimethyl formamide), have been synthesized and characterized by elemental analysis, IR and X-ray single-crystal diffraction. Compound 1 features a centrosymmetric tetranuclear copper cluster which further constructed a 1D chain through a tetra-acceptor hydrogen bonds of 4,4′-dpdo molecule. Compound 2 having a P21 /n space group also exhibits a tetranuclear nickel cluster with a cubane topology in which the central Ni(II) ion and oxygen atoms from H3L2− occupy the alternate vertices of the cube. Magnetic properties of 1 and 2 in the 2–300 K have also been discussed. The tetranuclear cubanes cores display dominant ferromagnetic interactions.







Similar content being viewed by others
References
Y. Xiao, Y. Q. Liu, G. Li, and P. Huang (2015). Supramol. Chem. 27, 161.
S.-H. Zhang, R.-X. Zhao, G. Li, H.-Y. Zhang, C.-L. Zhang, and G. Muller (2014). RSC Adv. 4, 54837.
P. A. Vigato, V. Peruzzo, and S. Tamburini (2012). Coord. Chem. Rev. 256, 953.
J. B. Pelayo-Vázquez, F. J. González, M. A. Leyva, M. Campos, L. A. Torres, and M. J. Rosales-Hoz (2012). J. Organomet. Chem. 716, 289.
L. J. Li, X. X. Hua, Y. Y. Huang, X. Y. Yang, C. Wang, and J. L. Du (2014). Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 44, 291.
R. A. Kumar, M. Arivanandhan, and Y. Hayakawa (2013). Prog. Cryst. Growth Charact. Mater. 59, 113.
Y. Zhao, K. Chen, J. Fan, T. Okamura, Y. Lu, L. Luo, and W. Y. Sun (2014). Dalton Trans. 43, 2252.
H. Fu, Y. Lu, Z. L. Wang, C. Liang, Z. M. Zhang, and E. Wang (2012). Dalton Trans. 41, 4084.
Y. Xiao, P. Huang, and W. Wang (2015). J. Clust. Sci. 26, 1091.
S. Hu, F. Y. Yu, P. Zhang, and D. R. Lin (2013). Dalton Trans. 42, 7731.
G. Bozoklu, C. Gateau, D. Imbert, J. Pécaut, K. Robeyns, Y. Filinchuk, F. Memon, G. Muller, and M. Mazzanti (2012). J. Am. Chem. Soc. 134, 8372.
P. Alborés and E. Rentschler (2009). Angew. Chem. Int. Ed. 48, 9366.
Y. Xiao, P. Huang, and Y. Q. Liu (2015). Mol. Cryst. Liq. Cryst. 607, 242.
L.-F. Ma, L.-Y. Wang, X.-K. Huo, Y.-Y. Wang, Y.-T. Fan, J.-G. Wang, and S.-H. Chen (2008). Cryst. Grow. Des. 8, 620.
T.-P. Hu, Z.-J. Xue, B.-H. Zheng, X.-Q. Wang, X.-N. Hao, and Y. Song (2016). CrystEngComm. 18, 5386.
J. Zhang, C. Zhang, Y. Xiao, Y. Qin, and S. Zhang (2016). Supramol. Chem. 28, 231.
J. P. Costes and L. Vendier (2010). Eur. J. Inorg. Chem. 2010, 2768.
P. L. Caradoc-Davies and L. R. Hanton (2001). Chem. Commun. 12, 1098.
S.-H. Zhang, M.-F. Tang, and C.-M. Ge (2009). Z. Anorg. Allg. Chem. 635, 1442.
G. M. Sheldrick (2008). Acta Cryst. A64, 112.
J. C. Pessoa, I. Cavaco, I. Correia, I. Tomaz, T. Duarte, and P. M. Matias (2000). J. Inorg. Biochem. 80, 35.
R. J. Butcher, Y. Gultneh, and K. Ayikoe (2009). Acta Cryst. E65, m1193.
H. J. Sun, L. She, S. M. Fang, and X. Y. Li (2008). Polyhedron 27, 854.
C. D. Papadopoulos, A. G. Hatzidimitriou, G. P. Voutsas, and A. Lalia-Kantouri (2007). Polyhedron 26, 1077.
S. Thakurta, P. Roy, R. J. Butcher, M. Salah El Fallah, J. Tercero, E. Garribba, and S. Mitra (2009). Eur. J. Inorg. Chem. 2009, 4385.
A. Banerjee, R. Singh, P. Mondal, E. Colacio, and K. K. Rajak (2010). Eur. J. Inorg. Chem. 2010, 790.
J. K. Eberhardt, T. Glaser, R.-D. Hoffmann, R. Fröhlich, and E.-U. Würthwein (2005). Eur. J. Inorg. Chem. 2005, 1175.
A. Sieber, C. Boskovic, R. Bircher, O. Waldmann, S. T. Ochsenbein, G. Chaboussant, H. U. Güdel, N. Kirchner, J. V. Slageren, W. Wernsdorfer, A. Neels, H. Stoeckli-Evans, S. Janssen, F. Juranyi, and H. Mutka (2005). Inorg. Chem. 44, 4315.
Y. Xiao, S.-H. Zhang, G.-Z. Li, Y.-G. Wang, and C. Feng (2011). Inorg. Chim. Acta 366, 39.
M. A. Halcrow, J. C. Huffman, and G. Christou (1995). Angew. Chem. Int. Ed. 34, 889.
S. Hazra, S. Mohanta, R. Koner, P. Lemoine, and E. C. Sañudo (2009). Eur. J. Inorg. Chem. 2009, 3458.
R. E. P. Winpenny (2002). J. Chem. Soc. Dalton Trans. 1, 1.
S. M. Aubin, N. R. Dilley, and L. Pardi (1998). J. Am. Chem. Soc. 120, 4991.
J. M. García-Lastra, M. T. Barriuso, and J. A. Aramburu (2005). J. Chem. Phys. 317, 103.
O. Kahn, J. Larionova, and J. V. Yakhmi (1999). Chem. Eur. J. 5, 3443.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (No. 21161006), the Nature Science Foundation of Guangxi Province of China (No. 2015GXNSFAA139031); Program for the scientific research and technology development plan of Guilin (No. 20150133-5); Guangxi Key Laboratory of Environmental Pollution Control Theory and Technology; the Innovation Project of Guangxi Graduate Education (SS201606).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Feng, C., Ge, C.M. et al. Two New Cubane-Type Tetranuclear Compounds of Copper(II), Nickel(II) Derived from Reduced Schiff Base Ligand: Syntheses, Structures and Magnetic Properties. J Clust Sci 27, 2001–2011 (2016). https://doi.org/10.1007/s10876-016-1064-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1064-1