Abstract
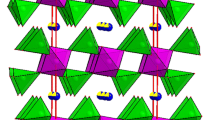
The sodium aluminum diphosphate compound has been synthesized by the classic ceramic method and characterized by X-ray diffraction technique, IR, 31P NMR, 23Na NMR and impedance spectroscopy. It crystallizes in monoclinic space group P21/c . The MAS-NMR spectra showed two and one isotropic resonances relatives to 31P and 23Na, respectively, revealing the existence of two phosphorus and one sodium environments in the structure. The electrical properties of this compound have been measured in the temperature range from 523 to 673 K and the frequency range from 209 Hz to 5 MHz. The Nyquist plots are fitted to an equivalent circuit modeled by a combination series of two parallel (R//C) and (R//CPE). Impedance measurements show NaAlP2O7 an ionic conductor being the conductivity 1.16 × 10−5 Ωcm−1 at 613 K and Ea is 0.95 eV. The conductivity provide nearly the same activation energies for electrical relaxation of mobile ions revealing that transport properties in this material appear to be due to an ionic hopping mechanism dominated by the motion of the Na+ ions along [101] tunnels direction presented in the structure of the investigated material. The peak positions ωp of M″ spectra shift toward higher frequencies with increase in temperature.













Similar content being viewed by others
References
H. Imai, Y. Kamiya, T Okuhara (2007). J. Catal. 251, 195.
M. Baril, H. Assaaoudi, J. A. Kozinski, and I. S, Butler (2007). Inorg. Chim. Acta. 360, 3155.
C. Parada, J. Perles, R. Saez-Puche, C. Ruiz-Valero, and N. Snejko (2003). Chem. Mater. 15, 3347.
S. Seyyidoglu, M. Ozenbas, N. Yazici, A. Yilmaz (2007). J. Mater. Sci. 42, 6453.
A. B. Rhaiem, S. Chouaib, K. Guidara (2010). Ionics. 16, 455.
M. Gabelica-Robert, M. Goreaud, P. Labbe, B. Raveau (1982). J. Solid State Chem. 45, 389.
J. P. Gamondes, F. d’Yvoire, A. Boulle (1971). C. R. Acad. Sci. 49, 272.
I. Grunze, H. Grunze, Z. Anorg (1984). Allg. Chem. 512, 39.
D. Riou, N. Nguyen, and R. Benloucif (1990). Mater. Res. Bull. 25, 1363.
S. Nasri, M. Megdiche, K. Guidara, M. Gargouri (2013). Ionics. 10, 969.
H. Nam Ng, C. Calvo (1973). Can. J. Chem. 51, 2613.
S. L. Wang, P. G. Wang, Y. P. Nieh (1990). J. Appl. Cryst. 23, 520.
D. Riou, N. Nguyen, R. Benloucif, B. Baveau (1990). Mater. Res. Bull. 25, 1363.
A. Leclaire, A. Benmoussa, N. M. Borel, A. Grandin, B. Raveau (1988). J. Solid State Chem. 77, 299.
A. Hamady, M. F. Zid, T. Jouini (1994). J. Solid State Chem. 113, 120.
A. Hamady, T. Jouini (1996). Acta. Cryst. C 52, 2949.
T. Shao-long, L. Yuan-Ying, Y. Yan-sheng (1997). J. Phys. Chem. Solids. 58, 957.
J. Alkemper, H. Paulus, H. Fuess (1994). Z. Kristallogr. 209, 616.
D. Massiot, H. Theile, and A. Germanius (1994). Bruker Rep. 43, 140.
M. Serghini Idrissi, L. Rghioui, R. Nejjar, L. Benarafa, M. Saidi Idrissi, A. Lorriaux, F. Wallart (2004). Spectrochim. Acta Part A. 60, 2043.
N. Khay, A. Ennaciri, M. Harcharras (2001). Vib. Spectrosc. 27, 119.
N. Khay, A. Ennaciri (2001). J. Alloys. Compd. 323, 800.
N. Khay, A. Ennaciri, A. Rulmont (2001). J. Raman Spectrosc. 32, 1052.
D. Massiot, H. Theile, A. Germanius (1994). Bruker Rep. 140, 43.
F. Hlel, S. Kamoun, K. Guidara (1994). Z. Naturforsch. 61(2006), 375.
H. Mahamoud, B. Louati, F. Hlel, K. Guidara (2011). J Alloys Compd. 509, 6083.
S. Chatterjee, P. K. Mahapatra, R. N. P. Choudhary, and A. K. Thakur (2004). Phys. Status Solidi A 201, 588.
H. Rahmouni, R. Jemai, N. Kallel, A. Selmi, K Khirouni (2010). J. Alloys Compd. 497, 1.
S. Nasri, M. Megdiche, K. Guidara, M Gargouri (2013). Ionics. 10, 927.
N. F. Mott and E. A. Davis Electronic Processes in Non-crystalline Materials, 2nd ed (Clarendon, Oxford, 1979). 225.
S. R. Elliott (1987). Adv. Phys. 36, 135.
B. V. R. Chowdari, R. Gopalakrishnan (1987). Solid State Ionics 23, 225.
S. Ghosh, A Ghosh (2002). Solid State Ionics 149, 67.
A. Oueslati, F. Hlel, K. Guidara, M Gargouri (2010). J. Alloys. Compd. 492, 508.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben Taher, Y., Hajji, R., Oueslati, A. et al. Infra-red, NMR Spectroscopy and Transport Properties of Diphosphate NaAlP2O7 . J Clust Sci 26, 1279–1294 (2015). https://doi.org/10.1007/s10876-014-0812-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-014-0812-3