Abstract
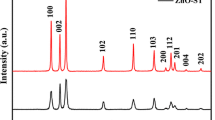
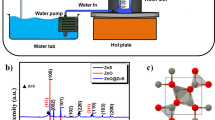
In this article, we report on the synthesis, characterization and photocatalytic activity of quasi spherical ZnO nanoparticles obtained by an egg white assisted facile sol–gel type wet method. The material was characterized for its structural, textural and optical properties. The hexagonal wurtzite crystalline structure of ZnO with high phase purity was confirmed by the X-ray diffraction analysis. The mesoporous texture generated from the inter-agglomeration of ZnO nanoparticles was clearly shown in the transmission electron microscopy (TEM) images. The N2 sorption analysis indicated a specific surface area of 18 m2/g, with monomodal mesoporosity. The optical studies had shown the decreased optical band gap (3.28 eV) of the sample with the existence of a number of crystal defects, especially oxygen vacancies in the sample. The aquatic dye pollutants were effectively degraded under UV irradiation over the ZnO nano photocatalysts. They were also found to be reusable up to five consecutive runs without loss in catalytic activity, indicating their high photostability against photocorrosion.











Similar content being viewed by others
References
S. A. Khayyat, M. Abaker, A. Umar, M. O. Alkattan, N. D. Alharbi, and S. Baskoutas (2012). J. Nanosci. Nanotechnol. 12, 8453.
Z. L. Wang (2008). ACS Nano 2, 1987.
A. Belaidi, Th Dittrich, D. Kieven, J. Tornow, K. Schwarzburg, M. Kunst, N. Allsop, M-Ch Lux-Steiner, and S. Gavrilov (2009). Sol. Energy Mater. Sol. Cells 93, 1033.
Q. Zhang, C. Xie, S. Zhang, A. Wang, B. Zhu, L. Wang, et al. (2005). Sens Actuators B 110, 370.
S. Z. Kang, T. Wu, X. Li, and J. Mu (2010). Colloids Surf. A. Eng. Aspects 369, 268.
T. Aoki, Y. Hatanaka, and D. C. Look (2000). Appl. Phys. Lett. 76, 3257.
D. Qian, J. Z. Jiang, and P. L. Hansen (2003). Chem. Commun. 9, 1078.
K. Vignesh, A. Suganthi, M. Rajarajan, and S. A. Sara (2012). Powder Technol. 224, 331.
J. L. Yang, S. J. An, W. I. Park, G. Y. Yi, and W. Choi (2004). Adv. Mater. 16, 1661.
L. Armelao, G. Bottaro, M. Pascolini, M. Sessolo, E. Tondello, M. Bettinelli, and A. Speghini (2008). J. Phys. Chem. C 112, 4049.
L. Y. Yang, S. Y. Dong, J. H. Sun, J. L. Feng, Q. H. Wu, and S. P. Sun (2010). J. Hazard. Mater. 179, 438.
S. A. Studenikin, N. Golego, and M. Cocivera (2000). J Appl Phys 87, 2413.
Z. W. Pan, Z. R. Dai, and Z. L. Wang (2001). Science 291, 1947.
P. X. Gao and Z. L. Wang (2004). Appl. Phys. Lett. 84, 2883.
Y. Liu, H. Lv, S. Li, X. Xing, and G. Xi (2012). Dyes Pigm. 95, 443.
M. Zareie, A. Gholami, M. Bahrami, A. H. Rezaei, and M. H. Keshavarz (2013). Mater Lett. 91, 255.
M. Pudukudy, A. Hetieqa, and Z. Yaakob (2014). Appl. Surf. Sci. doi:10.1016/j.apsusc.2014.07.050.
T. Ghoshal, S. Kar, and S. Chaudhuri (2007). Cryst. Growth Des. 7, 136.
M. Bitenc, P. Podbrscek, Z. C. Orel, M. A. Cleveland, J. A. Paramo, R. M. Peters, and Y. M. Strzhemechny (2009). Cryst. Growth Des. 9, 997.
D. Kim and Y. D. Huh (2011). Mater. Lett. 65, 2100.
M. S. Mohajerani, M. Mazloumi, A. Lak, A. Kajbafvala, S. Zanganeh, and S. K. Sadrnezhaad (2008). J. Cryst. Growth 310, 3621.
Y. Zhang, W. F. Zhang, and H. W. Zheng (2007). Scripta Mater. 57, 313.
Z. Liu, Z. Jin, W. Li, and J. Qiu (2005). Mater. Lett. 59, 3620.
D. Chen, X. Jiao, and G. Cheng (2000). Solid State Commun. 113, 363.
V. Bansal, P. Poddar, A. Ahmad, and M. Sastry (2006). J. Am. Chem. Soc. 128, 11958.
Y. L. Zou, Y. Li, J. G. Li, and W. J. Xie (2012). Chem. Pap. 66, 278.
D. V. Vadehra, K. R. Nath, and R. Forsythe (1973). CRC Crit. Rev. Food Technol. 4, 193.
S. Dhara and P. Bhargava (2001). J. Am. Chem. Soc. 84, 3048.
F. Nouroozi and F. Farzaneh (2011). J. Braz. Chem. Soc. 22, 484.
P. Thangaraj, J. Rajan, S. Durai, S. Kumar, A. R. Phanic, and G. Neri (2011). Vaccum 86, 140.
M. Shoeb, B. R. Singh, J. A Khan, W. Khan, B. N. Singh, H. B. Singh, and A. H. Naqv (2013). Adv. Nat. Sci. 4, 035015.
N. Daneshvar, S. Aber, M. S. Seyed Dorraji, A. R. Khatae, and M. H. Rasoulifard (2007). Sep. Purific. Technol. 58, 91.
M. Pudukudy and Z. Yaakob (2013). Superlattices Microstruct. 63, 47.
Z. Zhu, D. Yang, and H. Liu (2011). Adv. Powder Technol. 22, 493.
M. Pudukudy, Z. Yaakob, B. Narayanan, A. Gopalakrishnan, and S. M. Tasirin (2013). Superlattices Microstruct. 64, 15.
L. Sun, R. Shao, Z. Chen, L. Tang, Y. Dai, and J. Ding (2012). Appl. Surf. Sci. 258, 5455.
Q. F. Zhang, T. P. Chou, B. Russo, S. A. Jenekhe, and G. Z. Cao (2008). Angew. Chem. Int. Ed. 47, 2402.
T. Prakash, R. Jayaprakash, D. S. Raja, S. Kumar, N. Donatoc, D. Spadarod, and G. Neri (2013). Sens. Actuators B 176, 560.
L. J. Song, Z. Shuo, L. Z. Quan, Z. K. Jun, C. J. Kang, and Q. J.-Hao (2012) Trans. Nonferrous Met. Soc. China 22, 2459.
Q. Dong, H. Su, J. Xu, D. Zhang, and R. Wang (2007). Mater. Lett. 61, 2714.
M. Hedstrom, F. Plieva, I. Y. Galaev, and B. Mattiasson (2008). Anal. Bioanal. Chem. 390, 907.
A. J. Wooten, D. J. Werder, D. J. Williams, J. L. Casson, and J. A. Hollingsworth (2009). J Am Chem Soc 131, 16177.
J. H. Sun, S. Y. Dong, J. L. Feng, X.-J. Yin, and X. C. Zhao (2011). J. Mol. Catal. A 335, 145.
A. J. Reddy, M. K. Kokila, H. Nagabhushan, J. L. Rao, C. Shivakumar, B. M. Nagabhushan, and R. P. S. Chakradhar (2011). Spectrochim. Acta A 81, 53.
S. Baskoutas and G. Bester (2010). J. Phys. Chem. C 114, 9301.
S. Baskoutas and G. Bester (2011). J. Phys. Chem. C 115, 15862.
Z. Huang, D. Yan, M. Yang, X. Liao, Y. Kang, G. Yin, and Y. Yao (2008). J. Colloid Interface Sci. 325, 356.
U. Ozgur, Y. I. Alivov, C. Liu, A. Teke, M. A. Reshchikov, S. Dogan, V. Avrutin, S. J. Cho, and H. Morkoc (2005). J. Appl. Phys. 98, 041301.
K. Vanheusden, W. L. Warren, C. H. Seager, D. R. Tallant, J. A. Voigt, and B. E. Gnade (1996). J. Appl. Phys. 79, 7983.
M. Pudukudy and Z. Yaakob (2014). Solid State Sci. 30, 78.
J. Zhou, F. Zhao, Y. Wang, Y. Zhang, and L. Yang (2007). J. Lumin. 122–123, 195.
N. Kiomarsipourn and R. S. Razavi (2013). Ceram. Int. 39, 813.
G. Patrinoiu, M. Tudose, J. M. C. Moreno, R. Birjega, P. Budrugea, R. Ene, and O. Carp (2012). J. Solid State Chem. 186, 17.
I. Fatimah, S. Wang, and D. Wulandari (2011). Appl. Clay Sci. 53, 553.
A. Kajbafval, H. Ghorbani, A. Paravar, J. P. Samberg, E. Kajbafvala, and S. K. Sadrnezhaad (2012). Superlattices Microstruct. 51, 512.
Y. J. Xu, Y. B. Zhuang, and X. Z. Fu (2010). J. Phys. Chem. C 114, 2669.
K. Thongsuriwong, P. Amornpitoksuk, and S. Suwanboon (2013). Adv. Powd. Tech. 24, 275.
T. Warang, N. Patel, A. Santini, N. Bazzanella, and A. Kale (2012). Appl. Catal. A 423–424, 21.
M. Khatamian, A. A. Khandar, B. Divband, M. Haghighi, and S. Ebrahimiaslc (2012). J. Mol. Catal. A 365, 120.
M. Pudukudy and Z. Yaakob (2014). Appl. Surf. Sci. 292, 520.
M. Pudukudy, Z. Yaakob, R. Rajendran, and T. Kandaramath (2014). React. Kinet. Mech. Catal. 112, 527.
Q. Xiao and L. Ouyang (2009). J. Alloys Compd. 479, L4.
Acknowledgments
This work was financially supported by Yayasan Sime Darby (YSD), Universiti Kebangsaan Malaysia, under grant PKT/2012, Teknologi Sisa Sifar (331326006). The authors would like to acknowledge FST and CRIM for the material analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pudukudy, M., Yaakob, Z. Facile Synthesis of Quasi Spherical ZnO Nanoparticles with Excellent Photocatalytic Activity. J Clust Sci 26, 1187–1201 (2015). https://doi.org/10.1007/s10876-014-0806-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-014-0806-1