Abstract
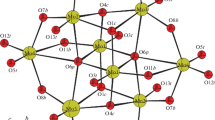
A new polyoxomolybdate (C6H6)4[H4GeMo12O40]·1.5H2O (1) has been isolated under hydrothermal condition and characterized by IR spectroscopy, elemental analysis and thermal stability. Single crystal X-ray structure analysis revealed that 1 crystallized in monoclinic space group P21/n with a = 10.9070(8) Å, b = 20.9582(11) Å, c = 11.4607(8) Å, β = 109.983(4)º, V = 2,462.1(3) Å3, Z = 2. The most significant feature of 1 is that the benzene rings are derived from the bis(carboxyethylgermanium) sesquoxide (H2E2Ge2O3) ligands via in situ reaction.
Graphical abstract
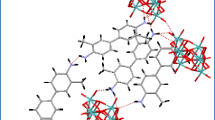
A new polyoxomolybdate (C6H6)4[H4GeMo12O40].1.5H2O (1) has been obtained from in situ reaction by using H2E2Ge2O3 ligands in the presence of molybdates. The formation of compound 1 involves the in situ reaction occurred in H2E2Ge2O3 ligand in the presence of molybdates via the doubly dehydrogenative coupling reaction.





Similar content being viewed by others
References
U. Kortz, M. G. Savelieff, F. Y. A. Ghali, L. M. Khalil, S. A. Maalouf, and D. Sinno (2002). Angew. Chem. Int. Ed. 41, 4070.
X.-Y. Wei, M. H. Dickman, and M. T. Pope (1998). J. Am. Chem. Soc. 120, 10254.
E. Antonova, B. Seidlhofer, J. Wang, M. Hinz, and W. Bensch (2012). Chem. Eur. J. 18, 15316.
S. Li, J. Zhao, P. Ma, J. Du, J. Niu, and J. Wang (2009). Inorg. Chem. 48, 9819.
M. Nyman, F. Bonhomme, T. M. Alam, J. B. Parise, and G. M. B. Vaughan (2004). Angew. Chem. Int. Ed. 43, 2787.
D. Pitzschke, J. Wang, R.-D. Hoffmann, R. Pöttgen, and W. Bensch (2006). Angew Chem. Int. Ed. 45, 1305.
J. Wang, C. Näther, P. Kögerler, and W. Bensch (2006). Eur. J. Inorg. Chem. 1237.
B. S. Bassil, M. H. Dickman, I. Römer, B. Kammer, and U. Kortz (2007). Angew. Chem. Int. Ed. 46, 1.
J.-W. Zhao, J. Zhang, S.-T. Zheng, and G.-Y. Yang (2008). Chem. Commun. 570.
U. Kortz, A. Müller, J. Slageren, J. Schnack, N. S. Dalal, and M. Dressel (2009). Coord. Chem. Rev. 253, 2315.
S.-T. Zheng, J. Zhang, J. M. Clemente-Juan, D.-Q. Yuan, and G.-Y. Yang (2009). Angew. Chem. Int. Ed. 48, 7176.
S.-T. Zheng, J. Zhang, and G.-Y. Yang (2008). Angew. Chem. Int. Ed. 47, 3909.
C. Wang, D. Yang, J. Wang, P. Ma, J. Wang, and J. Niu (2012). J. Mol. Struct. 1011, 1.
J.-P. Wang, X.-D. Du, and J.-Y. Niu (2006). J. Solid State Chem. 179, 3260.
H. Zhang, L. Duan, Y. Lan, E. Wang, and C. Hu (2003). Inorg. Chem. 42, 8053.
J. Sha, J. Peng, H. Liu, J. Chen, A. Tian, B. Dong, and P. Zhang (2008). J. Coord. Chem. 61, 1221.
H. He, G.-J. Cao, S.-T. Zheng, and G.-Y. Yang (2009). J. Am. Chem. Soc. 131, 15588.
G.-J. Cao, S.-T. Zheng, N. Zhao, J.-K. Sun, and G.-Y. Yang (2010). Inorg. Chem. 49, 10211.
N. Stock, C. Jargstorff, and S. Wriedt (2011). Z. Anorg. Allg. Chem. 637, 572.
C. Schmidt, A. Lieb, and N. Stock (2011). Z. Anorg. Allg. Chem. 637, 2163.
C. Schmidt and N. Stock (2011). Cryst. Growth Des. 11, 5682.
H.-F. Liu, R.-S. Liu, K. Y. Liew, R. E. Johnson, and J. H. Lunsford (1984). J. Am. Chem. Soc. 106, 4117.
Q. Li, Y. Wei, J. Hao, Y. Zhu, and L. Wang (2007). J. Am. Chem. Soc. 129, 5810.
G. M. Sheldrick SADABS, Program for Siemens Area Detector Absorption Corrections (University of Göttingen, Göttingen, 1997).
G. M. Sheldrick SHELXS97, Program for Crystal Structure Solution (University of Göttingen, Germany, 1997).
G. M. Sheldrick SHELXL97, Program for Crystal Structure Refinement (University of Göttingen, Germany, 1997).
K. Nakamoto Infrared spectra of inorganic and coordination compounds (Wiley, New York, 1970).
C.-Y. Pan, G.-Z. Liu, S.-T. Zheng, and G.-Y. Yang (2008). Chem. Eur. J. 14, 5057.
Acknowledgments
The authors are thankful for the financial supports from the the Natural Science Fund for Young Scholars of Fujian Province (Grant No. 2011J05018) and the Fund for Young Scholars from Fujian Agriculture and Forestry University (Grant No. 2011xjj06).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, GJ., Rong, C. (C6H6)4[H4GeMo12O40]·1.5H2O: A New Polyoxomolybdate Obtained from In Situ Reaction. J Clust Sci 24, 843–850 (2013). https://doi.org/10.1007/s10876-013-0581-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-013-0581-4